Influence of Chitin Source and Polymorphism on Powder Compression and Compaction: Application in Drug Delivery
- PMID: 33198143
- PMCID: PMC7697224
- DOI: 10.3390/molecules25225269
Influence of Chitin Source and Polymorphism on Powder Compression and Compaction: Application in Drug Delivery
Abstract
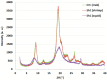
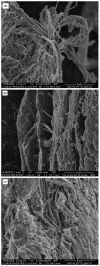
The objective of the research reported herein is to compare the compaction properties of three different chitin extracts from the organisms most used in the seafood industry; namely crabs, shrimps and squids. The foregoing is examined in relation to their polymorphic forms as well as compression and compaction behavior. Chitin extracted from crabs and shrimps exhibits the α-polymorphic form whilst chitin extracted from squid pins displays a β-polymorphic form. These polymorphs were characterized using FTIR, X-ray powder diffraction and scanning electron microscopy. Pore diameter and volume differ between the two polymorphic powder forms. The β form is smaller in pore diameter and volume. Scanning electron microscopy of the two polymorphic forms shows clear variation in the arrangement of chitin layers such that the α form appears more condensed due to the anti-parallel arrangement of the polymer chains. True, bulk and tapped densities of these polymorphs and their mixtures indicated poor flowability. Nevertheless, compression and compaction properties obtained by applying Heckle and Kawakita analyses indicated that both polymorphs are able to be compacted with differences in the extent of compaction. Chitin compacts, regardless of their origin, showed a very high crushing strength with very fast dissolution which makes them suitable for use as fast mouth dissolving tablets. Moreover, when different chitin powders are granulated with two model drugs, i.e., metronidazole and spiramycin they yielded high crushing strength and their dissolution profiles were in accordance with compendial requirements. It is concluded that the source of chitin extraction is as important as the polymorphic form when compression and compaction of chitin powders is carried out.
Keywords: chitin; chitin characterization; chitin sources; compression analysis; crushing strength; disintegration; dissolution; polymorphs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
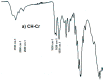
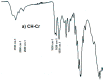







References
-
- Roberts G.A.F. Chitin Chemistry. Palgrave Macmillan Press Ltd.; London, UK: 1992. pp. 54–115.
-
- Budavari S., editor. The Merck Index. 13th ed. Merck and Co.; Kenilworth, NJ, USA: 2001.
-
- Uragami T., Tokura S. Material Science of Chitin and Chitosan. Springer; Berlin, Germany: Kodansha; Tokyo, Japan: 2006.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

