Respiratory Burst Oxidase Homologs RBOHD and RBOHF as Key Modulating Components of Response in Turnip Mosaic Virus- Arabidopsis thaliana (L.) Heyhn System
- PMID: 33198167
- PMCID: PMC7696843
- DOI: 10.3390/ijms21228510
Respiratory Burst Oxidase Homologs RBOHD and RBOHF as Key Modulating Components of Response in Turnip Mosaic Virus- Arabidopsis thaliana (L.) Heyhn System
Abstract
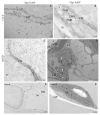
Turnip mosaic virus (TuMV) is one of the most important plant viruses worldwide. It has a very wide host range infecting at least 318 species in over 43 families, such as Brassicaceae, Fabaceae, Asteraceae, or Chenopodiaceae from dicotyledons. Plant NADPH oxidases, the respiratory burst oxidase homologues (RBOHs), are a major source of reactive oxygen species (ROS) during plant-microbe interactions. The functions of RBOHs in different plant-pathogen interactions have been analyzed using knockout mutants, but little focus has been given to plant-virus responses. Therefore, in this work we tested the response after mechanical inoculation with TuMV in ArabidopsisrbohD and rbohF transposon knockout mutants and analyzed ultrastructural changes after TuMV inoculation. The development of the TuMV infection cycle was promoted in rbohD plants, suggesting that RbohD plays a role in the Arabidopsis resistance response to TuMV. rbohF and rbohD/F mutants display less TuMV accumulation and a lack of virus cytoplasmic inclusions were observed; these observations suggest that RbohF promotes viral replication and increases susceptibility to TuMV. rbohD/F displayed a reduction in H2O2 but enhanced resistance similarly to rbohF. This dominant effect of the rbohF mutation could indicate that RbohF acts as a susceptibility factor. Induction of hydrogen peroxide by TuMV was partially compromised in rbohD mutants whereas it was almost completely abolished in rbohD/F, indicating that these oxidases are responsible for most of the ROS produced in this interaction. The pattern of in situ H2O2 deposition after infection of the more resistant rbohF and rbohD/F genotypes suggests a putative role of these species on systemic signal transport. The ultrastructural localization and quantification of pathogenesis-related protein 1 (PR1) indicate that ROS produced by these oxidases also influence PR1 distribution in the TuMV-A.thaliana pathosystem. Our results revealed the highest activation of PR1 in rbohD and Col-0. Thus, our findings indicate a correlation between PR1 accumulation and susceptibility to TuMV. The specific localization of PR1 in the most resistant genotypes after TuMV inoculation may indicate a connection of PR1 induction with susceptibility, which may be characteristic for this pathosystem. Our results clearly indicate the importance of NADPH oxidases RbohD and RbohF in the regulation of the TuMV infection cycle in Arabidopsis. These findings may help provide a better understanding of the mechanisms modulating A.thaliana-TuMV interactions.
Keywords: hydrogen peroxide; pathogenesis-related protein 1; plant–virus interaction; potyvirus; resistance response; ultrastructure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Revers F., García J.A. Molecular biology of potyviruses. Adv. Virus Res. 2015;92:101–199. - PubMed
-
- Liu X.P., Lu W.C., Liu Y.K., Li J.L. A study of TuMV strain differentiation of cruciferous vegetables from ten provinces of China. Chin. Sci. Bull. 1990;35:1734–1739.
-
- Yoon J.Y., Green S.K., Opena R.T. Inheritance of resistance to Turnip mosaic virus in Chinese cabbage. Euphytica. 1993;69:103–108. doi: 10.1007/BF00021732. - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

