Recent Advances in the Biological Investigation of Organometallic Platinum-Group Metal (Ir, Ru, Rh, Os, Pd, Pt) Complexes as Antimalarial Agents
- PMID: 33198217
- PMCID: PMC7698227
- DOI: 10.3390/molecules25225276
Recent Advances in the Biological Investigation of Organometallic Platinum-Group Metal (Ir, Ru, Rh, Os, Pd, Pt) Complexes as Antimalarial Agents
Abstract
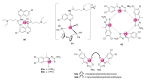
In the face of the recent pandemic and emergence of infectious diseases of viral origin, research on parasitic diseases such as malaria continues to remain critical and innovative methods are required to target the rising widespread resistance that renders conventional therapies unusable. The prolific use of auxiliary metallo-fragments has augmented the search for novel drug regimens in an attempt to combat rising resistance. The development of organometallic compounds (those containing metal-carbon bonds) as antimalarial drugs has been exemplified by the clinical development of ferroquine in the nascent field of Bioorganometallic Chemistry. With their inherent physicochemical properties, organometallic complexes can modulate the discipline of chemical biology by proffering different modes of action and targeting various enzymes. With the beneficiation of platinum group metals (PGMs) in mind, this review aims to describe recent studies on the antimalarial activity of PGM-based organometallic complexes. This review does not provide an exhaustive coverage of the literature but focusses on recent advances of bioorganometallic antimalarial drug leads, including a brief mention of recent trends comprising interactions with biomolecules such as heme and intracellular catalysis. This resource can be used in parallel with complementary reviews on metal-based complexes tested against malaria.
Keywords: Plasmodium falciparum; bioorganometallic chemistry; malaria; mechanism of action; platinum-group metals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures















Similar articles
-
Enhancing the Activity of Drugs by Conjugation to Organometallic Fragments.Chemistry. 2020 Jul 17;26(40):8676-8688. doi: 10.1002/chem.201904699. Epub 2020 May 26. Chemistry. 2020. PMID: 32452579 Free PMC article. Review.
-
A Structural Chemistry Perspective on the Antimalarial Properties of Thiosemicarbazone Metal Complexes.Mini Rev Med Chem. 2019;19(7):569-590. doi: 10.2174/1389557518666181015152657. Mini Rev Med Chem. 2019. PMID: 30324878 Review.
-
Structure-activity relationship studies of antiplasmodial cyclometallated ruthenium(II), rhodium(III) and iridium(III) complexes of 2-phenylbenzimidazoles.Eur J Med Chem. 2019 Jan 1;161:11-21. doi: 10.1016/j.ejmech.2018.10.019. Epub 2018 Oct 10. Eur J Med Chem. 2019. PMID: 30342422
-
Metal containing chloroquinolines: beyond hit and miss antimalarial efficacy to solid science.Mini Rev Med Chem. 2013 Apr;13(4):597-606. doi: 10.2174/1389557511313040011. Mini Rev Med Chem. 2013. PMID: 22974366 Review.
-
Antimicrobial evaluation of neutral and cationic iridium(III) and rhodium(III) aminoquinoline-benzimidazole hybrid complexes.Eur J Med Chem. 2020 Nov 15;206:112694. doi: 10.1016/j.ejmech.2020.112694. Epub 2020 Aug 5. Eur J Med Chem. 2020. PMID: 32861176
Cited by
-
Facing Diseases Caused by Trypanosomatid Parasites: Rational Design of Pd and Pt Complexes With Bioactive Ligands.Front Chem. 2022 Jan 7;9:816266. doi: 10.3389/fchem.2021.816266. eCollection 2021. Front Chem. 2022. PMID: 35071192 Free PMC article. Review.
-
A theoretical study on the on-off phosphorescence of novel Pt(ii)/Pt(iv)-bisphenylpyridinylmethane complexes.RSC Adv. 2022 Jun 22;12(28):18238-18244. doi: 10.1039/d2ra03060h. eCollection 2022 Jun 14. RSC Adv. 2022. PMID: 35800316 Free PMC article.
-
Synthesis, characterization, and antibacterial activities of a heteroscorpionate derivative platinum complex against methicillin-resistant Staphylococcus aureus.Front Cell Infect Microbiol. 2023 Mar 27;13:1100947. doi: 10.3389/fcimb.2023.1100947. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37051297 Free PMC article.
-
Exploring the antimalarial and antioxidant efficacy of transition metal(II) chelates of thiosemicarbazone ligands: spectral investigations, molecular docking, DFT, MESP and ADMET.Biometals. 2024 Feb;37(1):247-265. doi: 10.1007/s10534-023-00546-1. Epub 2023 Nov 8. Biometals. 2024. PMID: 37938497
-
Experimental and computational evaluation of anti-malarial and antioxidant potential of transition metal (II) complexes with tridentate schiff base derived from pyrrolopyrimidine.Biometals. 2024 Dec;37(6):1713-1737. doi: 10.1007/s10534-024-00636-8. Epub 2024 Sep 13. Biometals. 2024. PMID: 39271604
References
-
- Global Polio Eradication Initiative Applauds WHO African Region for Wild Polio-Free Certification. [(accessed on 15 October 2020)]; Available online: https://www.who.int/news/item/25-08-2020-global-polio-eradication-initia....
-
- Malaria. [(accessed on 15 October 2020)]; Available online: https://www.who.int/news-room/fact-sheets/detail/malaria.
-
- World Health Organization . World Malaria Report 2018. World Health Organization; Geneva, Switzerland: 2019.
-
- World Health Organization . Status Report on Artemisinin Resistance. World Health Organization; Geneva, Switzerland: 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

