Mechanical, Histological, and Scanning Electron Microscopy Study of the Effect of Mixed-Acid and Heat Treatment on Additive-Manufactured Titanium Plates on Bonding to the Bone Surface
- PMID: 33198250
- PMCID: PMC7696444
- DOI: 10.3390/ma13225104
Mechanical, Histological, and Scanning Electron Microscopy Study of the Effect of Mixed-Acid and Heat Treatment on Additive-Manufactured Titanium Plates on Bonding to the Bone Surface
Abstract
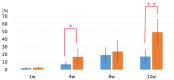
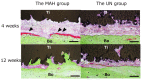
The additive manufacturing (AM) technique has attracted attention as one of the fully customizable medical material technologies. In addition, the development of new surface treatments has been investigated to improve the osteogenic ability of the AM titanium (Ti) plate. The purpose of this study was to evaluate the osteogenic activity of the AM Ti with mixed-acid and heat (MAH) treatment. Fully customized AM Ti plates were created with a curvature suitable for rat calvarial bone, and they were examined in a group implanted with the MAH-treated Ti in comparison with the untreated (UN) group. The AM Ti plates were fixed to the surface of rat calvarial bone, followed by extraction of the calvarial bone 1, 4, 8, and 12 weeks after implantation. The bonding between the bone and Ti was evaluated mechanically. In addition, AM Ti plates removed from the bone were examined histologically by electron microscopy and Villanueva-Goldner stain. The mechanical evaluation showed significantly stronger bone-bonding in the MAH group than in the UN group. In addition, active bone formation was seen histologically in the MAH group. Therefore, these findings indicate that MAH resulted in rapid and strong bonding between cortical bone and Ti.
Keywords: additive manufacturing; bone bonding; custom-made plate; mixed-acid and heat treatment; selective laser melting; titanium.
Conflict of interest statement
The authors declare that no conflict of interest exists.
Figures






Similar articles
-
Histological Evaluation of Porous Additive-Manufacturing Titanium Artificial Bone in Rat Calvarial Bone Defects.Materials (Basel). 2021 Sep 17;14(18):5360. doi: 10.3390/ma14185360. Materials (Basel). 2021. PMID: 34576584 Free PMC article.
-
Osteogenic capacity of mixed-acid and heat-treated titanium mesh prepared by a selective laser melting technique.RSC Adv. 2018 Jul 20;8(46):26069-26077. doi: 10.1039/c8ra04193h. eCollection 2018 Jul 19. RSC Adv. 2018. PMID: 35541945 Free PMC article.
-
Enhanced biomechanical performance of additively manufactured Ti-6Al-4V bone plates.J Mech Behav Biomed Mater. 2021 Jul;119:104552. doi: 10.1016/j.jmbbm.2021.104552. Epub 2021 Apr 23. J Mech Behav Biomed Mater. 2021. PMID: 33934037
-
Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution.Acta Biomater. 2020 Jun;109:1-20. doi: 10.1016/j.actbio.2020.03.037. Epub 2020 Apr 6. Acta Biomater. 2020. PMID: 32268239 Review.
-
A Review of the Applications of Additive Manufacturing Technologies Used to Fabricate Metals in Implant Dentistry.J Prosthodont. 2020 Aug;29(7):579-593. doi: 10.1111/jopr.13212. Epub 2020 Jun 25. J Prosthodont. 2020. PMID: 32548890 Review.
Cited by
-
Bilayer Membrane Composed of Mineralized Collagen and Chitosan Cast Film Coated With Berberine-Loaded PCL/PVP Electrospun Nanofiber Promotes Bone Regeneration.Front Bioeng Biotechnol. 2021 Jul 19;9:684335. doi: 10.3389/fbioe.2021.684335. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34350160 Free PMC article.
-
Bioactivation Treatment with Mixed Acid and Heat on Titanium Implants Fabricated by Selective Laser Melting Enhances Preosteoblast Cell Differentiation.Nanomaterials (Basel). 2021 Apr 12;11(4):987. doi: 10.3390/nano11040987. Nanomaterials (Basel). 2021. PMID: 33921268 Free PMC article.
-
Histological Evaluation of Porous Additive-Manufacturing Titanium Artificial Bone in Rat Calvarial Bone Defects.Materials (Basel). 2021 Sep 17;14(18):5360. doi: 10.3390/ma14185360. Materials (Basel). 2021. PMID: 34576584 Free PMC article.
References
-
- US Food and Drug Administration . Technical Considerations for AM Medical Devices. US Food and Drug Administration; Concord, MD, USA: 2017.
Grants and funding
LinkOut - more resources
Full Text Sources

