Pharmacogenomics for Primary Care: An Overview
- PMID: 33198260
- PMCID: PMC7696803
- DOI: 10.3390/genes11111337
Pharmacogenomics for Primary Care: An Overview
Abstract
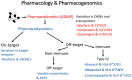
Most of the prescribing and dispensing of medicines happens in primary care. Pharmacogenomics (PGx) is the study and clinical application of the role of genetic variation on drug response. Mounting evidence suggests PGx can improve the safety and/or efficacy of several medications commonly prescribed in primary care. However, implementation of PGx has generally been limited to a relatively few academic hospital centres, with little adoption in primary care. Despite this, many primary healthcare providers are optimistic about the role of PGx in their future practice. The increasing prevalence of direct-to-consumer genetic testing and primary care PGx studies herald the plausible gradual introduction of PGx into primary care and highlight the changes needed for optimal translation. In this article, the potential utility of PGx in primary care will be explored and on-going barriers to implementation discussed. The evidence base of several drug-gene pairs relevant to primary care will be outlined with a focus on antidepressants, codeine and tramadol, statins, clopidogrel, warfarin, metoprolol and allopurinol. This review is intended to provide both a general introduction to PGx with a more in-depth overview of elements relevant to primary care.
Keywords: adverse drug reaction; antidepressants; clopidogrel; drug hypersensitivity; implementation; pharmacogenomics; primary care; statins; warfarin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gray K.A., Yates B., Seal R.L., Wright M.W., Bruford E.A. Genenames.org: The HGNC resources in 2015. Nucleic Acids Res. 2015;43:1079–1085. doi: 10.1093/nar/gku1071. - DOI - PMC - PubMed
-
- Wei M.Y., Ito M.K., Cohen J.D., Brinton E.A., Jacobson T.A. Predictors of statin adherence, switching, and discontinuation in the USAGE survey: Understanding the use of statins in America and gaps in patient education. J. Clin. Lipidol. 2013;7:472–483. doi: 10.1016/j.jacl.2013.03.001. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

