Emerging Strategies to Combat β-Lactamase Producing ESKAPE Pathogens
- PMID: 33198306
- PMCID: PMC7697847
- DOI: 10.3390/ijms21228527
Emerging Strategies to Combat β-Lactamase Producing ESKAPE Pathogens
Abstract
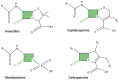
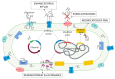
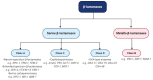
Since the discovery of penicillin by Alexander Fleming in 1929 as a therapeutic agent against staphylococci, β-lactam antibiotics (BLAs) remained the most successful antibiotic classes against the majority of bacterial strains, reaching a percentage of 65% of all medical prescriptions. Unfortunately, the emergence and diversification of β-lactamases pose indefinite health issues, limiting the clinical effectiveness of all current BLAs. One solution is to develop β-lactamase inhibitors (BLIs) capable of restoring the activity of β-lactam drugs. In this review, we will briefly present the older and new BLAs classes, their mechanisms of action, and an update of the BLIs capable of restoring the activity of β-lactam drugs against ESKAPE (Enterococcus spp., Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) pathogens. Subsequently, we will discuss several promising alternative approaches such as bacteriophages, antimicrobial peptides, nanoparticles, CRISPR (clustered regularly interspaced short palindromic repeats) cas technology, or vaccination developed to limit antimicrobial resistance in this endless fight against Gram-negative pathogens.
Keywords: ESKAPE; antimicrobial resistance; inhibitors; vaccination; β-lactamase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Otero L.H., Rojas-Altuve A., Llarrull L.I., Carrasco-López C., Kumarasiri M., Lastochkin E., Fishovitz J., Dawley M., Hesek D., Lee M., et al. How allosteric control of Staphylococcus aureus penicillin binding protein 2a enables methicillin resistance and physiological function. Proc. Natl. Acad. Sci. USA. 2013;110:16808–16813. doi: 10.1073/pnas.1300118110. - DOI - PMC - PubMed
-
- Gonzales P.R., Pesesky M.W., Bouley R., Ballard A., Biddy B.A., Suckow M.A., Wolter W.R., Schroeder V.A., Burnham C.A.D., Mobashery S., et al. Synergistic, collaterally sensitive β-lactam combinations suppress resistance in MRSA. Nat. Chem. Biol. 2015;11:855–861. doi: 10.1038/nchembio.1911. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

