Photosensitizers Based on G-Quadruplex Ligand for Cancer Photodynamic Therapy
- PMID: 33198362
- PMCID: PMC7697063
- DOI: 10.3390/genes11111340
Photosensitizers Based on G-Quadruplex Ligand for Cancer Photodynamic Therapy
Abstract
G-quadruplex (G4) is the non-canonical secondary structure of DNA and RNA formed by guanine-rich sequences. G4-forming sequences are abundantly located in telomeric regions and in the promoter and untranslated regions (UTR) of cancer-related genes, such as RAS and MYC. Extensive research has suggested that G4 is a potential molecular target for cancer therapy. Here, we reviewed G4 ligands as photosensitizers for cancer photodynamic therapy (PDT), which is a minimally invasive therapeutic approach. The photosensitizers, such as porphyrins, were found to be highly toxic against cancer cells via the generation of reactive oxidative species (ROS) upon photo-irradiation. Several porphyrin derivatives and analogs, such as phthalocyanines, which can generate ROS upon photo-irradiation, have been reported to act as G4 ligands. Therefore, they have been implicated as promising photosensitizers that can selectively break down cancer-related DNA and RNA forming G4. In this review, we majorly focused on the potential application of G4 ligands as photosensitizers, which would provide a novel strategy for PDT, especially molecularly targeted PDT (mtPDT).
Keywords: G-quadruplex; RAS; cancer; photodynamic therapy; photosensitizer; telomeres.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures



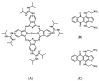

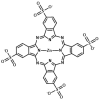
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

