Long-term cost-utility analysis of remote monitoring of older patients with pacemakers: the PONIENTE study
- PMID: 33198629
- PMCID: PMC7670660
- DOI: 10.1186/s12877-020-01883-3
Long-term cost-utility analysis of remote monitoring of older patients with pacemakers: the PONIENTE study
Abstract
Background: Cost-effectiveness studies on pacemakers have increased in the last years. However the number of long-term cost-utility studies is limited. The objective of this study was to perform a cost-utility analysis comparing remote monitoring (RM) versus conventional monitoring (CM) in hospital of older patients with pacemakers, 5 years after implant.
Methods: Under a controlled, not randomized, nor masked clinical trial, 83 patients with pacemakers were initially selected. After five years of follow-up, a total of 55 patients (CM = 34; RM = 21) completed the study. A cost-utility analysis of RM in terms of costs per gained quality-adjusted life years (QALYs) was conducted. The costs from the Public Health System (PHS) as well as patients and their relatives were taken into account for the study. The robustness of the results was verified by the probabilistic analyses through Monte-Carlo simulations.
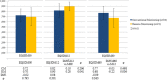
Results: After a five-year follow-up period, total costs were lower in the RM group by 23.02% than in the CM group (€274.52 versus €356.62; p = 0.033) because of a cost saving from patients' perspective (€59.05 versus €102.98; p = 0.002). However, the reduction of in-hospital visits derived from RM exhibited insignificant impact on the costs from the PHS perspective, with a cost saving of 15.04% (€215.48 vs. €253.64; p = 0.144). Costs/QALYs obtained by the RM group were higher as compared to the CM group, although there were no significant differences. The incremental cost-effectiveness ratio of CM in comparison to RM became positive (€301.16).
Conclusions: This study confirms RM of older patients with pacemakers appears still as a cost-utility alternative to CM in hospital after 5 years of follow-up.
Trial registration: ClinicalTrials.gov: (Identifier: NCT02234245 ). Registered 09 September 2014 - Prospectively registered.
Keywords: Cost-utility; Pacemakers follow-up; Quality-adjusted life years; Remote monitoring; Telemedicine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, Hayes DL, Howlett JG, Kautzner J, Love CJ, Morgan JM, Priori SG, Reynolds DW, Schoenfeld MH, Vardas PE, Heart Rhythm Society (HRS); European Heart Rhythm Association (EHRA); American College of Cardiology (ACC); American Heart Association (AHA); European Society of Cardiology (ESC); Heart Failure Association of ESC (HFA); Heart Failure Society of America (HFSA). HRS/EHRA Expert Consensus on the Monitoring of Cardiovascular Implantable Electronic Devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations: developed in partnership with the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA); and in collaboration with the American College of Cardiology (ACC), the American Heart Association (AHA), the European Society of Cardiology (ESC), the Heart Failure Association of ESC (HFA), and the Heart Failure Society of America (HFSA) Endorsed by the Heart Rhythm Society, the European Heart Rhythm Association (a registered branch of the ESC), the American College of Cardiology, the American Heart Association. Europace. 2008;10(6):707–725. doi: 10.1093/europace/eun122. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical