PD-L1 and B7-1 Cis-Interaction: New Mechanisms in Immune Checkpoints and Immunotherapies
- PMID: 33199209
- PMCID: PMC7914151
- DOI: 10.1016/j.molmed.2020.10.004
PD-L1 and B7-1 Cis-Interaction: New Mechanisms in Immune Checkpoints and Immunotherapies
Abstract
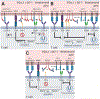
Immune checkpoints negatively regulate immune cell responses. Programmed cell death protein 1:programmed death ligand 1 (PD-1:PD-L1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4):B7-1 are among the most important immune checkpoint pathways, and are key targets for immunotherapies that seek to modulate the balance between stimulatory and inhibitory signals to lead to favorable therapeutic outcomes. The current dogma of these two immune checkpoint pathways has regarded them as independent with no interactions. However, the newly characterized PD-L1:B7-1 ligand-ligand cis-interaction and its ability to bind CTLA-4 and CD28, but not PD-1, suggests that these pathways have significant crosstalk. Here, we propose that the PD-L1:B7-1 cis-interaction brings novel mechanistic understanding of these pathways, new insights into mechanisms of current immunotherapies, and fresh ideas to develop better treatments in a variety of therapeutic settings.
Keywords: B7-1; CD28; CTLA-4; PD-1; PD-L1; cis-interaction; immunotherapy.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors declare no competing financial interests.
Figures


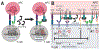
References
-
- Ledford H, et al. (2018) Cancer immunologists scoop medicine Nobel prize. Nature 562, 20–21 - PubMed
-
- van Nimwegen JF, et al. (2020) Abatacept treatment for patients with early active primary Sjögren’s syndrome: a single-centre, randomised, double-blind, placebo-controlled, phase 3 trial (ASAP-III study). Lancet Rheumatol 2, e153–e163 - PubMed
-
- Kliwinski C, et al. (2005) Prophylactic administration of abatacept prevents disease and bone destruction in a rat model of collagen-induced arthritis. J Autoimmun 25, 165–171 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

