Impact of patient characteristics on efficacy and safety of once-weekly semaglutide versus dulaglutide: SUSTAIN 7 post hoc analyses
- PMID: 33199417
- PMCID: PMC7670946
- DOI: 10.1136/bmjopen-2020-037883
Impact of patient characteristics on efficacy and safety of once-weekly semaglutide versus dulaglutide: SUSTAIN 7 post hoc analyses
Abstract
Objective: In SUSTAIN 7, once-weekly semaglutide demonstrated superior glycated haemoglobin (HbA1c) and body weight (BW) reductions versus once-weekly dulaglutide in subjects with type 2 diabetes (T2D). This post hoc analysis investigated the impact of clinically relevant subject characteristics on treatment effects of semaglutide versus dulaglutide.
Design: Analyses by baseline age (<65, ≥65 years), sex (male, female), diabetes duration (≤5, >5-10, >10 years), HbA1c (≤7.5, >7.5-8.5, >8.5% (≤58, >58-69, >69 mmol/mol)) and body mass index (BMI) (<30, 30-<35, ≥35 kg/m2).
Setting: 194 sites; 16 countries.
Participants: Subjects with T2D (n=1199) exposed to treatment.
Interventions: Semaglutide 0.5 mg versus dulaglutide 0.75 mg (low-dose comparison); semaglutide 1.0 mg versus dulaglutide 1.5 mg (high-dose comparison), all subcutaneously once weekly.
Primary and secondary outcome measures: Change in HbA1c (primary endpoint) and BW (confirmatory secondary endpoint) from baseline to week 40; proportion of subjects achieving HbA1c targets (<7%, ≤6.5% (<53, ≤48 mmol/mol)) and weight-loss responses (≥5%, ≥10%) at week 40; and safety.
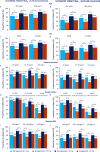
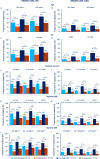
Results: HbA1c and BW reductions (estimated treatment difference ranges: -0.22 to -0.70%-point; -1.76 to -3.84 kg) and proportion of subjects achieving HbA1c targets and weight-loss responses were statistically significantly greater for the majority of comparisons of semaglutide versus dulaglutide within each subgroup category and, excepting glycaemic control within the low-dose comparison in HbA1c subgroups, this was irrespective of subgroup or dose comparison. Gastrointestinal adverse events, the most common with both treatments, were reported by more women than men and, with semaglutide, decreased with increasing BMI.
Conclusions: Consistently greater improvements in HbA1c and BW with semaglutide versus dulaglutide, regardless of age, sex, diabetes duration, glycaemic control and BMI, support the efficacy of semaglutide across the continuum of care in a heterogeneous population with T2D.
Trial registration number: NCT02648204.
Keywords: clinical trials; diabetes & endocrinology; general diabetes.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The authors received no grants or funding for the writing of this article. In relation to the submitted work, RP reports grants from Novo Nordisk to his institution, AdventHealth, a nonprofit organisation, and VA also reports grants from Novo Nordisk to her institution.Outside of this work, RP reports speaker and consulting fees from AstraZeneca; consulting fees from Boehringer Ingelheim; consulting fees from Eisai, Inc.; consulting fees from GlaxoSmithKline; consulting fees from Glytec, LLC; consulting fees from Janssen; grants from Lexicon Pharmaceuticals; grants and consulting fees from Ligand Pharmaceuticals, Inc;, grants and consulting fees from Lilly; grants and consulting fees from Merck; consulting fees from Mundipharma; grants, speaker fees and consulting fees from Novo Nordisk; consulting fees from Pfizer; grants and consulting fees from Sanofi; grants, speaker fees and consulting fees from Takeda; and personal consulting fees from Sanofi US Services, Inc. Except for consulting fees in February 2018 and June 2018 from Sanofi US Services, Inc., RP’s services were paid for directly to AdventHealth. VA has received consulting fees (to her institution) from Adocia; grants (to her institution) and consulting fees (to her institution) from AstraZeneca/BMS; consulting fees from Becton-Dickinson; grants (to her institution) from Boehringer Ingelheim; grants (to her institution) from Calibra; consulting fees from Duke University; grants (to her institution) from Eisai; grants (to her institution) from Fractyl; grants (to her institution) from Janssen; grants (to her institution) and consulting fees from Novo Nordisk; grants (to her institution) and consulting fees from Sanofi; grants (to her institution) from Theracos; and consulting fees from Zafgen, all outside of the submitted work; and is the spouse of an employee of Merck Research Laboratories. AMC is an employee ofNovo Nordisk. In relation to the submitted work, IL reports grants to her institution from Novo Nordisk. Outside of the submitted work, IL has received consulting fees from AstraZeneca; consulting fees from Boehringer Ingelheim; grants from GI Dynamics; consulting fees from Intarcia; consulting fees from Janssen; consulting fees from Lilly; consulting fees from Mannkind; grants from Merck; grants from Mylan; grants from Novartis; grants and consulting fees from Novo Nordisk; grants from Pfizer; consulting fees from Sanofi; consultant fees from TARGETPharma; and consulting fees from Valeritas, all outside of the submitted work. JL reports being an investigator in clinical studies for Eli Lilly and Novo Nordisk outside of the submitted work. EY was, at the time of writing the manuscript, an employee and minor shareholder of Novo Nordisk. AV has received lecture/other fees and non-financial (clinical research) support from AstraZeneca; non-financial (clinical research) support from Amgen; lecture/other fees from Boehringer Ingelheim; lecture/other fees and non-financial (clinical research) support from Eli Lilly; lecture/other fees fromMerck Sharp & Dohme; lecture/other fees from Napp, lecture/other fees and non-financial (clinical research) support from Novo Nordisk; non-financial (clinical research) support from Novartis; and lecture/other fees and non-financial (clinical research) support from Sanofi, all outside of the submitted work.
Figures

References
-
- American Diabetes Association . Standards of medical care in Diabetes–2019. Diabetes Care 2019;42(Suppl1):S1–2. - PubMed
-
- Dennis JM, Shields BM, Henley WE, et al. . Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol 2019;7:442–51. 10.1016/S2213-8587(19)30087-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
