Defining the Lowest Threshold for Amyloid-PET to Predict Future Cognitive Decline and Amyloid Accumulation
- PMID: 33199430
- PMCID: PMC7905788
- DOI: 10.1212/WNL.0000000000011214
Defining the Lowest Threshold for Amyloid-PET to Predict Future Cognitive Decline and Amyloid Accumulation
Abstract
Introduction: As clinical trials move toward earlier intervention, we sought to redefine the β-amyloid (Aβ)-PET threshold based on the lowest point in a baseline distribution that robustly predicts future Aβ accumulation and cognitive decline in 3 independent samples of clinically normal individuals.
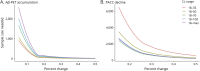
Methods: Sequential Aβ cutoffs were tested to identify the lowest cutoff associated with future change in cognition (Preclinical Alzheimer Cognitive Composite [PACC]) and Aβ-PET in clinically normal participants from the Harvard Aging Brain Study (n = 342), Australian Imaging, Biomarker and Lifestyle study of aging (n = 157), and Alzheimer's Disease Neuroimaging Initiative (n = 356).
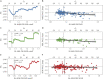
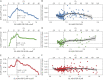
Results: Within samples, cutoffs derived from future Aβ-PET accumulation and PACC decline converged on the same inflection point, beyond which trajectories diverged from normal. Across samples, optimal cutoffs fell within a short range (Centiloid 15-18.5).
Discussion: These optimized thresholds can help to inform future research and clinical trials targeting early Aβ. Threshold convergence raises the possibility of contemporaneous early changes in Aβ and cognition.
Classification of evidence: This study provides Class II evidence that among clinically normal individuals a specific Aβ-PET threshold is predictive of cognitive decline.
© 2020 American Academy of Neurology.
Figures

References
-
- Klunk WE, Engler H, Nordberg A, et al. . Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound-B. Ann Neurol 2004;55:306–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EB014894/EB/NIBIB NIH HHS/United States
- K24 AG035007/AG/NIA NIH HHS/United States
- P30 AG066512/AG/NIA NIH HHS/United States
- S10 RR021110/RR/NCRR NIH HHS/United States
- P01 AG036694/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- U01 AG032438/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- R01 AG034556/AG/NIA NIH HHS/United States
- R21 AG038994/AG/NIA NIH HHS/United States
- R01 AG027435/AG/NIA NIH HHS/United States
- S10 RR023043/RR/NCRR NIH HHS/United States
- R01 AG037497/AG/NIA NIH HHS/United States
- U19 AG010483/AG/NIA NIH HHS/United States
- R01 AG026484/AG/NIA NIH HHS/United States
- P41 EB015896/EB/NIBIB NIH HHS/United States
- R01 AG062559/AG/NIA NIH HHS/United States
- S10 RR023401/RR/NCRR NIH HHS/United States
- K99 AG061238/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
