Canadian best practice recommendations for the management of amyotrophic lateral sclerosis
- PMID: 33199452
- PMCID: PMC7683000
- DOI: 10.1503/cmaj.191721
Canadian best practice recommendations for the management of amyotrophic lateral sclerosis
Conflict of interest statement
Competing interests: Christen Shoesmith, Aaron Izenberg, Wendy Johnston, Colleen O’Connell, Kerri Schellenberg and Lorne Zinman were members of a scientific advisory committee for Radicava (edaravone; Mitsubishi Tanabe Pharma Canada). Christen Shoesmith reports being a site principal investigator for several multicentre amyotrophic lateral sclerosis (ALS) clinical trials. In the last 36 months, Dr. Shoesmith has participated in clinical trials sponsored by Biogen, Cytokinetics, ALS Pharma and Orphazyme. Marvin Chum reports receiving a grant from Bernice Ramsay ALS Clinical Research Fellowship, outside the submitted work. Aaron Izenberg reports receiving personal fees from Biogen, Roche, Alnylam, Genzyme, Takeda and Mitsubishi Tanabe Pharma, outside the submitted work. No other competing interests were declared.
Figures
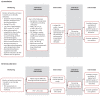
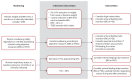
References
-
- Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med 2017;377:162–72. - PubMed
-
- van Es MA, Hardiman O, Chio A, et al. Amyotrophic lateral sclerosis. Lancet 2017;390:2084–98. - PubMed
-
- Shoesmith C. A recipe for ALS. Can J Neurol Sci 2008;35:125–126. - PubMed
-
- Health Canada approves new drug to treat patients with amyotrophic lateral sclerosis (ALS) [press release]. Ottawa: Health Canada; 2018. October 4.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
