Thymus-derived B cell clones persist in the circulation after thymectomy in myasthenia gravis
- PMID: 33199596
- PMCID: PMC7720237
- DOI: 10.1073/pnas.2007206117
Thymus-derived B cell clones persist in the circulation after thymectomy in myasthenia gravis
Abstract
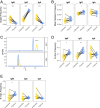
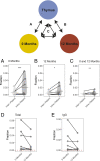
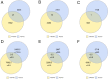
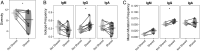
Myasthenia gravis (MG) is a neuromuscular, autoimmune disease caused by autoantibodies that target postsynaptic proteins, primarily the acetylcholine receptor (AChR) and inhibit signaling at the neuromuscular junction. The majority of patients under 50 y with AChR autoantibody MG have thymic lymphofollicular hyperplasia. The MG thymus is a reservoir of plasma cells that secrete disease-causing AChR autoantibodies and although thymectomy improves clinical scores, many patients fail to achieve complete stable remission without additional immunosuppressive treatments. We speculate that thymus-associated B cells and plasma cells persist in the circulation after thymectomy and that their persistence could explain incomplete responses to resection. We studied patients enrolled in a randomized clinical trial and used complementary modalities of B cell repertoire sequencing to characterize the thymus B cell repertoire and identify B cell clones that resided in the thymus and circulation before and 12 mo after thymectomy. Thymus-associated B cell clones were detected in the circulation by both mRNA-based and genomic DNA-based sequencing. These antigen-experienced B cells persisted in the circulation after thymectomy. Many circulating thymus-associated B cell clones were inferred to have originated and initially matured in the thymus before emigration from the thymus to the circulation. The persistence of thymus-associated B cells correlated with less favorable changes in clinical symptom measures, steroid dose required to manage symptoms, and marginal changes in AChR autoantibody titer. This investigation indicates that the diminished clinical response to thymectomy is related to persistent circulating thymus-associated B cell clones.
Keywords: B cells; adaptive immune cell receptor repertoire sequencing; autoimmune disease; myasthenia gravis; thymectomy.
Conflict of interest statement
Competing interest statement: K.C.O. has received research support from Ra Pharma and is a consultant and equity shareholder of Cabaletta Bio. K.C.O. is the recipient of a sponsored research subaward from the University of Pennsylvania, the primary financial sponsor of which is Cabaletta Bio. S.H.K. receives consulting fees from Northrop Grumman. R.J.N. has received research support from Alexion Pharmaceuticals, Genentech, Grifols, and Ra Pharma. H.J.K. has served as an advisor to Alnylam Pharmaceuticals, Ra Pharmaceuticals, and UCB Pharmaceuticals, and is Chief Exectutive Officer and Chief Marketing Officer of ARC Biotechnology, LLC, based on US Patent 8,961,98.
Figures
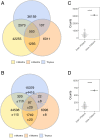
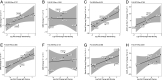
Comment in
-
Thymectomy and myasthenia gravis.Proc Natl Acad Sci U S A. 2020 Dec 22;117(51):32195-32196. doi: 10.1073/pnas.2022901117. Epub 2020 Dec 3. Proc Natl Acad Sci U S A. 2020. PMID: 33273117 Free PMC article. No abstract available.
References
-
- Vincent A., Unravelling the pathogenesis of myasthenia gravis. Nat. Rev. Immunol. 2, 797–804 (2002). - PubMed
-
- Gilhus N. E., et al. , Myasthenia gravis—Autoantibody characteristics and their implications for therapy. Nat. Rev. Neurol. 12, 259–268 (2016). - PubMed
-
- Lindstrom J. M., Engel A. G., Seybold M. E., Lennon V. A., Lambert E. H., Pathological mechanisms in experimental autoimmune myasthenia gravis. II. Passive transfer of experimental autoimmune myasthenia gravis in rats with anti-acetylcholine recepotr antibodies. J. Exp. Med. 144, 739–753 (1976). - PMC - PubMed
-
- Oda K., Korenaga S., Ito Y., Myasthenia gravis: Passive transfer to mice of antibody to human and mouse acetylcholine receptor. Neurology 31, 282–287 (1981). - PubMed
-
- Sterz R., et al. , Effector mechanisms in myasthenia gravis: End-plate function after passive transfer of IgG, Fab, and F(ab′)2 hybrid molecules. Muscle Nerve 9, 306–312 (1986). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

