The intracellular Ca2+ release channel TRPML1 regulates lower urinary tract smooth muscle contractility
- PMID: 33199609
- PMCID: PMC7720193
- DOI: 10.1073/pnas.2016959117
The intracellular Ca2+ release channel TRPML1 regulates lower urinary tract smooth muscle contractility
Abstract
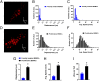
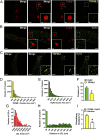
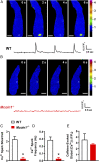
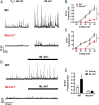
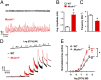
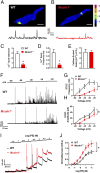
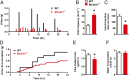
TRPML1 (transient receptor potential mucolipin 1) is a Ca2+-permeable, nonselective cation channel that is predominantly localized to the membranes of late endosomes and lysosomes (LELs). Intracellular release of Ca2+ through TRPML1 is thought to be pivotal for maintenance of intravesicular acidic pH as well as the maturation, fusion, and trafficking of LELs. Interestingly, genetic ablation of TRPML1 in mice (Mcoln1-/- ) induces a hyperdistended/hypertrophic bladder phenotype. Here, we investigated this phenomenon further by exploring an unconventional role for TRPML1 channels in the regulation of Ca2+-signaling activity and contractility in bladder and urethral smooth muscle cells (SMCs). Four-dimensional (4D) lattice light-sheet live-cell imaging showed that the majority of LELs in freshly isolated bladder SMCs were essentially immobile. Superresolution microscopy revealed distinct nanoscale colocalization of LEL-expressing TRPML1 channels with ryanodine type 2 receptors (RyR2) in bladder SMCs. Spontaneous intracellular release of Ca2+ from the sarcoplasmic reticulum (SR) through RyR2 generates localized elevations of Ca2+ ("Ca2+ sparks") that activate plasmalemmal large-conductance Ca2+-activated K+ (BK) channels, a critical negative feedback mechanism that regulates smooth muscle contractility. This mechanism was impaired in Mcoln1-/- mice, which showed diminished spontaneous Ca2+ sparks and BK channel activity in bladder and urethra SMCs. Additionally, ex vivo contractility experiments showed that loss of Ca2+ spark-BK channel signaling in Mcoln1-/- mice rendered both bladder and urethra smooth muscle hypercontractile. Voiding activity analyses revealed bladder overactivity in Mcoln1-/- mice. We conclude that TRPML1 is critically important for Ca2+ spark signaling, and thus regulation of contractility and function, in lower urinary tract SMCs.
Keywords: calcium signaling; endolysosomes; ion channels; lower urinary tract; superresolution microscopy.
Copyright © 2020 the Author(s). Published by PNAS.
Figures

Comment in
-
Igniting Ca2+ sparks with TRPML1.Proc Natl Acad Sci U S A. 2020 Dec 29;117(52):32836-32838. doi: 10.1073/pnas.2022896117. Epub 2020 Dec 1. Proc Natl Acad Sci U S A. 2020. PMID: 33262276 Free PMC article. No abstract available.
References
-
- Montell C., The TRP superfamily of cation channels. Sci. STKE 2005, re3 (2005). - PubMed
-
- Sun M., et al. , Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum. Mol. Genet. 9, 2471–2478 (2000). - PubMed
-
- Lindvall J. M., Blomberg K. E., Wennborg A., Smith C. I., Differential expression and molecular characterisation of Lmo7, Myo1e, Sash1, and Mcoln2 genes in Btk-defective B-cells. Cell. Immunol. 235, 46–55 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

