Exo1 recruits Cdc5 polo kinase to MutLγ to ensure efficient meiotic crossover formation
- PMID: 33199619
- PMCID: PMC7720183
- DOI: 10.1073/pnas.2013012117
Exo1 recruits Cdc5 polo kinase to MutLγ to ensure efficient meiotic crossover formation
Abstract
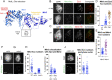
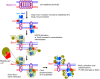
Crossovers generated during the repair of programmed meiotic double-strand breaks must be tightly regulated to promote accurate homolog segregation without deleterious outcomes, such as aneuploidy. The Mlh1-Mlh3 (MutLγ) endonuclease complex is critical for crossover resolution, which involves mechanistically unclear interplay between MutLγ and Exo1 and polo kinase Cdc5. Using budding yeast to gain temporal and genetic traction on crossover regulation, we find that MutLγ constitutively interacts with Exo1. Upon commitment to crossover repair, MutLγ-Exo1 associate with recombination intermediates, followed by direct Cdc5 recruitment that triggers MutLγ crossover activity. We propose that Exo1 serves as a central coordinator in this molecular interplay, providing a defined order of interaction that prevents deleterious, premature activation of crossovers. MutLγ associates at a lower frequency near centromeres, indicating that spatial regulation across chromosomal regions reduces risky crossover events. Our data elucidate the temporal and spatial control surrounding a constitutive, potentially harmful, nuclease. We also reveal a critical, noncatalytic role for Exo1, through noncanonical interaction with polo kinase. These mechanisms regulating meiotic crossovers may be conserved across species.
Keywords: MutL; crossovers; meiosis; polo kinase; recombination.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Allers T., Lichten M., Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106, 47–57 (2001). - PubMed
-
- Hunter N., Kleckner N., The single-end invasion: An asymmetric intermediate at the double-strand break to double-Holliday junction transition of meiotic recombination. Cell 106, 59–70 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

