Detection of SARS-CoV-2 antibodies is insufficient for the diagnosis of active or cured COVID-19
- PMID: 33199713
- PMCID: PMC7669901
- DOI: 10.1038/s41598-020-76914-5
Detection of SARS-CoV-2 antibodies is insufficient for the diagnosis of active or cured COVID-19
Abstract
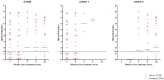
We assessed the performance of Abbott's SARS-CoV-2 IgG assay and the PanbioTM COVID-19 IgG/IgM rapid test device for the diagnosis of either active or cured COVID-19. Three cohorts of patients were chosen. Cohort 1, patients (n = 65) who attended the emergency department on March 30, 2020 with clinical suspicion of active COVID-19 (n = 56 with proven/probable COVID-19). Cohort 2, hospital workers (n = 92) who had either been (n = 40) or not (n = 52) diagnosed with proven/probable COVID-19 and were asymptomatic at the time of the sampling. Cohort 3, patients (n = 38) cared at the hospital before the start of the COVID-19 pandemic. Detection of serum antibodies was done using Abbott´s SARS-CoV-2 IgG assay and the PanbioTM COVID-19 IgG/IgM device. Both methods showed 98% agreement for IgG detection. No antibodies were detected in the 38 samples from hospitalized pre-COVID subjects. The diagnostic performance of IgGs detected by Abbott´s SARS-CoV-2 assay in Cohorts 1/2 was: sensitivity (60.7%/75%) and specificity (100%/84.6%). The diagnostic performance of IgM by PanbioTM COVID-19 in Cohorts 1/2 was: sensitivity (16%/17.5%) and specificity (100%/98.1%). We show that IgG detection alone is insufficient for the diagnosis of active or cured COVID-19. IgM detection has a limited diagnostic value.
Conflict of interest statement
The authors declares no competing interest within the scope of this manuscript. This study did not receive any funding. PE (MS15/00115) is a recipient of a Miguel Servet contract supported by FIS (Fondo de Investigación Sanitaria, Instituto de Salud Carlos III). JG is a steady researcher contracted by Fundación para la Investigación Biomédica del Hospital Gregorio Marañón. AAU (CM18/0089) is supported by a Río Hortega contract supported by the FIS.
Figures
References
-
- Organization, W. H. Coronavirus disease (COVID-19) situation report – 134. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2... (2nd of June, 2020).
-
- Organization, W. H. Novel Coronavirus – China. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en (12th of January, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

