Potential virus-mediated nitrogen cycling in oxygen-depleted oceanic waters
- PMID: 33199808
- PMCID: PMC8115048
- DOI: 10.1038/s41396-020-00825-6
Potential virus-mediated nitrogen cycling in oxygen-depleted oceanic waters
Abstract
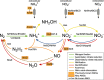
Viruses play an important role in the ecology and biogeochemistry of marine ecosystems. Beyond mortality and gene transfer, viruses can reprogram microbial metabolism during infection by expressing auxiliary metabolic genes (AMGs) involved in photosynthesis, central carbon metabolism, and nutrient cycling. While previous studies have focused on AMG diversity in the sunlit and dark ocean, less is known about the role of viruses in shaping metabolic networks along redox gradients associated with marine oxygen minimum zones (OMZs). Here, we analyzed relatively quantitative viral metagenomic datasets that profiled the oxygen gradient across Eastern Tropical South Pacific (ETSP) OMZ waters, assessing whether OMZ viruses might impact nitrogen (N) cycling via AMGs. Identified viral genomes encoded six N-cycle AMGs associated with denitrification, nitrification, assimilatory nitrate reduction, and nitrite transport. The majority of these AMGs (80%) were identified in T4-like Myoviridae phages, predicted to infect Cyanobacteria and Proteobacteria, or in unclassified archaeal viruses predicted to infect Thaumarchaeota. Four AMGs were exclusive to anoxic waters and had distributions that paralleled homologous microbial genes. Together, these findings suggest viruses modulate N-cycling processes within the ETSP OMZ and may contribute to nitrogen loss throughout the global oceans thus providing a baseline for their inclusion in the ecosystem and geochemical models.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures