Eliciting B cell immunity against infectious diseases using nanovaccines
- PMID: 33199883
- PMCID: PMC7855692
- DOI: 10.1038/s41565-020-00790-3
Eliciting B cell immunity against infectious diseases using nanovaccines
Abstract
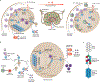
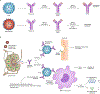
Infectious diseases, including the coronavirus disease 2019 (COVID-19) pandemic that has brought the world to a standstill, are emerging at an unprecedented rate with a substantial impact on public health and global economies. For many life-threatening global infectious diseases, such as human immunodeficiency virus (HIV) infection, malaria and influenza, effective vaccinations are still lacking. There are numerous roadblocks to developing new vaccines, including a limited understanding of immune correlates of protection to these global infections. To induce a reproducible, strong immune response against difficult pathogens, sophisticated nanovaccine technologies are under investigation. In contrast to conventional vaccines, nanovaccines provide improved access to lymph nodes, optimal packing and presentation of antigens, and induction of a persistent immune response. This Review provides a perspective on the global trends in emerging nanoscale vaccines for infectious diseases and describes the biological, experimental and logistical problems associated with their development, and how immunoengineering can be leveraged to overcome these challenges.
Conflict of interest statement
Competing interests
A.S. holds stocks or shares in Moderna, Inc.
Figures
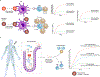
References
-
- Dawood FS et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect. Dis 12, 687–695 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

