Cholesterol access in cellular membranes controls Hedgehog signaling
- PMID: 33199907
- PMCID: PMC7872078
- DOI: 10.1038/s41589-020-00678-2
Cholesterol access in cellular membranes controls Hedgehog signaling
Abstract
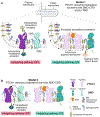
The Hedgehog (Hh) signaling pathway coordinates cell-cell communication in development and regeneration. Defects in this pathway underlie diseases ranging from birth defects to cancer. Hh signals are transmitted across the plasma membrane by two proteins, Patched 1 (PTCH1) and Smoothened (SMO). PTCH1, a transporter-like tumor-suppressor protein, binds to Hh ligands, but SMO, a G-protein-coupled-receptor family oncoprotein, transmits the Hh signal across the membrane. Recent structural, biochemical and cell-biological studies have converged at the surprising model that a specific pool of plasma membrane cholesterol, termed accessible cholesterol, functions as a second messenger that conveys the signal between PTCH1 and SMO. Beyond solving a central puzzle in Hh signaling, these studies are revealing new principles in membrane biology: how proteins respond to and remodel cholesterol accessibility in membranes and how the cholesterol composition of organelle membranes is used to regulate protein function.
Figures

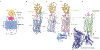

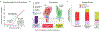


Similar articles
-
Patched 1 reduces the accessibility of cholesterol in the outer leaflet of membranes.Elife. 2021 Oct 26;10:e70504. doi: 10.7554/eLife.70504. Elife. 2021. PMID: 34698632 Free PMC article.
-
Measuring and Manipulating Membrane Cholesterol for the Study of Hedgehog Signaling.Methods Mol Biol. 2022;2374:73-87. doi: 10.1007/978-1-0716-1701-4_7. Methods Mol Biol. 2022. PMID: 34562244 Free PMC article.
-
Cholesterol Modification of Smoothened Is Required for Hedgehog Signaling.Mol Cell. 2017 Apr 6;66(1):154-162.e10. doi: 10.1016/j.molcel.2017.02.015. Epub 2017 Mar 23. Mol Cell. 2017. PMID: 28344083
-
Structures of vertebrate Patched and Smoothened reveal intimate links between cholesterol and Hedgehog signalling.Curr Opin Struct Biol. 2019 Aug;57:204-214. doi: 10.1016/j.sbi.2019.05.015. Epub 2019 Jun 24. Curr Opin Struct Biol. 2019. PMID: 31247512 Free PMC article. Review.
-
Hedgehog signaling and its molecular perspective with cholesterol: a comprehensive review.Cell Mol Life Sci. 2022 Apr 29;79(5):266. doi: 10.1007/s00018-022-04233-1. Cell Mol Life Sci. 2022. PMID: 35486193 Free PMC article. Review.
Cited by
-
Palmitoylation of Hedgehog proteins by Hedgehog acyltransferase: roles in signalling and disease.Open Biol. 2021 Mar;11(3):200414. doi: 10.1098/rsob.200414. Epub 2021 Mar 3. Open Biol. 2021. PMID: 33653085 Free PMC article. Review.
-
Sterols, Oxysterols, and Accessible Cholesterol: Signalling for Homeostasis, in Immunity and During Development.Front Physiol. 2021 Oct 8;12:723224. doi: 10.3389/fphys.2021.723224. eCollection 2021. Front Physiol. 2021. PMID: 34690800 Free PMC article. Review.
-
The Role of Sonic Hedgehog in Human Holoprosencephaly and Short-Rib Polydactyly Syndromes.Int J Mol Sci. 2021 Sep 12;22(18):9854. doi: 10.3390/ijms22189854. Int J Mol Sci. 2021. PMID: 34576017 Free PMC article. Review.
-
Differential responses to maternal diabetes in embryo and visceral yolk sac.Front Cell Dev Biol. 2023 Oct 19;11:1273641. doi: 10.3389/fcell.2023.1273641. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37928898 Free PMC article.
-
Paradoxes: Cholesterol and Hypoxia in Preeclampsia.Biomolecules. 2024 Jun 13;14(6):691. doi: 10.3390/biom14060691. Biomolecules. 2024. PMID: 38927094 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

