CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels
- PMID: 33199925
- PMCID: PMC8752031
- DOI: 10.1038/s42255-020-00298-z
CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels
Abstract
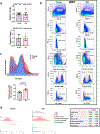
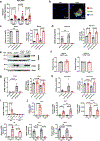
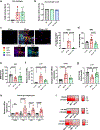
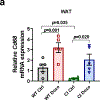
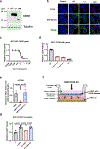
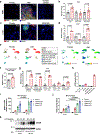
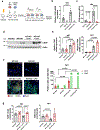
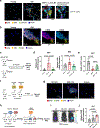
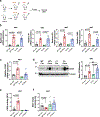
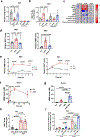
Decreased NAD+ levels have been shown to contribute to metabolic dysfunction during aging. NAD+ decline can be partially prevented by knockout of the enzyme CD38. However, it is not known how CD38 is regulated during aging, and how its ecto-enzymatic activity impacts NAD+ homeostasis. Here we show that an increase in CD38 in white adipose tissue (WAT) and the liver during aging is mediated by accumulation of CD38+ immune cells. Inflammation increases CD38 and decreases NAD+. In addition, senescent cells and their secreted signals promote accumulation of CD38+ cells in WAT, and ablation of senescent cells or their secretory phenotype decreases CD38, partially reversing NAD+ decline. Finally, blocking the ecto-enzymatic activity of CD38 can increase NAD+ through a nicotinamide mononucleotide (NMN)-dependent process. Our findings demonstrate that senescence-induced inflammation promotes accumulation of CD38 in immune cells that, through its ecto-enzymatic activity, decreases levels of NMN and NAD+.
Conflict of interest statement
Competing Interests Statement
Dr. Chini holds a patent on the use of CD38 inhibitors for metabolic diseases that is licensed by Elysium health. E.N.C is a consultant for TeneoBio, Calico, Mitobridge and Cyokinetics. E.N.C is on the advisory board of Eolo Pharma (Santa Fe, Argentina) W.v.S. is the Chief Scientific Officer of Teneobio, a company interested in the development of therapeutic antibodies. W.v.S, K.D., E.N.C, and S.C own stocks of Teneobio. J.L.K., T.T., J.M.vD., and D.J.B. have a financial interest related to this research: patents on transgenic animals capable of being induced to delete senescent cells are held by Mayo Clinic. J.L.K., T.T., D.J.B. and J.M.v.D. are co-inventors on patent applications licensed to or filed by Unity Biotechnology, a company developing senolytic medicines, including small molecules that selectively eliminate senescent cells. J.M.v.D. is a co-founder of Unity Biotechnology. J.M.S. is a cofounder of Transposon Therapeutics, Inc., serves as Chair of its Scientific Advisory Board, and consults for Astellas Innovation Management LLC, Atropos Therapeutics, Inc. and Gilead Sciences, Inc. J.D.R. is a member of the Rutgers Cancer Institute of New Jersey and of the University of Pennsylvania Diabetes Research Center; a co-founder and stockholder in VL54, Sofro, and Raze Therapeutics; and advisor and stockholder in Agios Pharmaceuticals, Kadmon Pharmaceuticals, Bantam Pharmaceuticals, Colorado Research Partners, Rafael Pharmaceuticals, and L.E.A.F. Pharmaceuticals. J.B. has intellectual property related to the use of NAD precursors in liver regeneration. All other authors declare no conflict of interest. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest policies.
Figures








References
Methods-only references
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

