Colorectal liver metastases: Current management and future perspectives
- PMID: 33200074
- PMCID: PMC7643190
- DOI: 10.5306/wjco.v11.i10.761
Colorectal liver metastases: Current management and future perspectives
Abstract
The liver is the commonest site of metastatic disease for patients with colorectal cancer, with at least 25% developing colorectal liver metastases (CRLM) during the course of their illness. The management of CRLM has evolved into a complex field requiring input from experienced members of a multi-disciplinary team involving radiology (cross sectional, nuclear medicine and interventional), Oncology, Liver surgery, Colorectal surgery, and Histopathology. Patient management is based on assessment of sophisticated clinical, radiological and biomarker information. Despite incomplete evidence in this very heterogeneous patient group, maximising resection of CRLM using all available techniques remains a key objective and provides the best chance of long-term survival and cure. To this end, liver resection is maximised by the use of downsizing chemotherapy, optimisation of liver remnant by portal vein embolization, associating liver partition and portal vein ligation for staged hepatectomy, and combining resection with ablation, in the context of improvements in the functional assessment of the future remnant liver. Liver resection may safely be carried out laparoscopically or open, and synchronously with, or before, colorectal surgery in selected patients. For unresectable patients, treatment options including systemic chemotherapy, targeted biological agents, intra-arterial infusion or bead delivered chemotherapy, tumour ablation, stereotactic radiotherapy, and selective internal radiotherapy contribute to improve survival and may convert initially unresectable patients to operability. Currently evolving areas include biomarker characterisation of tumours, the development of novel systemic agents targeting specific oncogenic pathways, and the potential re-emergence of radical surgical options such as liver transplantation.
Keywords: Cancer; Colorectal; Liver; Management; Metastases; Review.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Figures
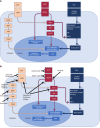

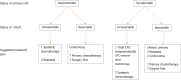
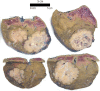
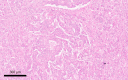

References
-
- Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–1480. - PubMed
-
- Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–1649. - PubMed
-
- Skipworth RJ, Parks RW, Stephens NA, Graham C, Brewster DH, Garden OJ, Paterson-Brown S. The relationship between hospital volume and post-operative mortality rates for upper gastrointestinal cancer resections: Scotland 1982-2003. Eur J Surg Oncol. 2010;36:141–147. - PubMed
-
- Cecil TD, Sexton R, Moran BJ, Heald RJ. Total mesorectal excision results in low local recurrence rates in lymph node-positive rectal cancer. Dis Colon Rectum. 2004;47:1145–9; discussion 1149-50. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

