Preventive Efficacy of Tenofovir/Emtricitabine Against Severe Acute Respiratory Syndrome Coronavirus 2 Among Pre-Exposure Prophylaxis Users
- PMID: 33200081
- PMCID: PMC7543639
- DOI: 10.1093/ofid/ofaa455
Preventive Efficacy of Tenofovir/Emtricitabine Against Severe Acute Respiratory Syndrome Coronavirus 2 Among Pre-Exposure Prophylaxis Users
Abstract
Background: The preventive effect that tenofovir/emtricitabine (FTC) could have against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human immunodeficiency virus-negative people is unknown. The objective of this study was to analyze the seroprevalence and clinical manifestations of COVID-19 among users of pre-exposure prophylaxis (PrEP), disoproxil fumarate/FTC (TDF/FTC), or tenofovir alafenamide (TAF)/FTC and to compare it to that of a control group.
Methods: An observational descriptive study of the seroprevalence of antibodies for SARS-CoV-2 among men who have sex with men and transgender women without use of PrEP (Group 1; n = 250) and PrEP users with TDF/FTC (n = 409) or TAF/FTC (n = 91) (Group 2; n = 500) was conducted from May11, 2020 to June 27, 2020. All participants were provided with a structured questionnaire that collected information on the variables to be analyzed, and testing for immunoglobulin G antibodies to SARS-CoV-2 (chemiluminescent microparticle immunoassay) was then carried out.
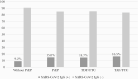
Results: The seroprevalence of SARS-CoV-2 was 9.2% (95% confidence interval [CI], 5.9-13.5) in the group without PrEP and 15.0% (95% CI, 12.0-18.4) in the group with PrEP (P = .026). Among users of TDF/FTC it was 14.7% (95% CI, 11.4-18.5), and in users of TAF/FTC it was 16.5% (95% CI, 9.5-25.7) (P = .661). In those who tested positive for SARS-CoV-2 and receiving PrEP, 57.4% manifested symptoms, compared with 78.3% in the control group (P = .070). In users of TDF/FTC the figure was 53.3% and in users of TAF/FTC the figure was 73.3% (P = .100). The duration of symptoms was 11.5 days in the control group, 9.0 days in PrEP users (P = .116), 7.0 days in users of TDF/FTC, and 13.0 days in users of TAF/FTC (P = .100).
Conclusions: Users of PrEP, TDF/FTC, or TAF/FTC presented a higher seroprevalence to SARS-CoV-2 than the control group. No statistically significant differences were found in relation to clinical manifestations. The PrEP users should use the same prevention measures as those indicated for the general population.
Keywords: COVID-19; SARS-CoV-2; disoproxil fumarate/emtricitabine (TDF/FTC); pre-exposure prophylaxis (PrEP); tenofovir alafenamide/emtricitabine (TAF/FTC).
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA 2020; 323:709–10. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

