Prothrombotic hemostasis disturbances in patients with severe COVID-19: Individual daily data
- PMID: 33200086
- PMCID: PMC7654236
- DOI: 10.1016/j.dib.2020.106519
Prothrombotic hemostasis disturbances in patients with severe COVID-19: Individual daily data
Abstract
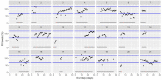
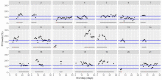
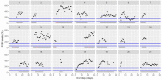
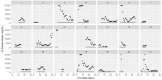
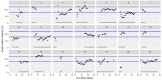
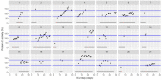
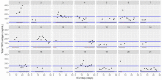
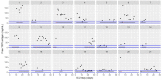
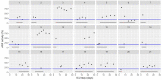
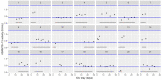
This data article accompanies the manuscript entitled: "Prothrombotic Disturbances of hemostasis of Patients with Severe COVID-19: a Prospective Longitudinal Observational Cohort Study" submitted to Thrombosis Research by the same authors. We report temporal changes of plasma levels of an extended set of laboratory parameters during the ICU stay of the 21 COVID-19 patients included in the monocentre cohort: CRP, platelet count, prothrombin time; Clauss fibrinogen and clotting factors II, V and VIII levels, D-dimers, antithrombin activity, protein C, free protein S, total and free tissue factor pathway inhibitor, PAI-1 levels, von Willebrand factor antigen and activity, ADAMTS-13 (plasma levels); and of two integrative tests of coagulation (thrombin generation with ST Genesia) and fibrinolysis (global fibrinolytic capacity - GFC). Regarding hemostasis, we used double-centrifuged frozen citrated plasma prospectively collected after daily performance of usual coagulation tests. Demographic and clinical characteristics of patients and thrombotic and hemorrhagic complications were also collected from patient's electronic medical reports.
Keywords: COVID-19; D-dimers; Fibrinolysis; Hemostasis plasma proteins; Intensive care unit; Thrombin generation; Thrombosis.
© 2020 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that have or could be perceived to have influenced the work reported in this article.
Figures
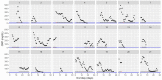
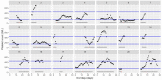
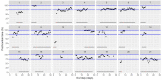
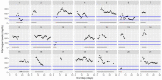
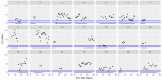

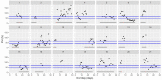

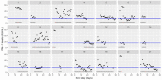
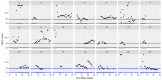


Similar articles
-
Prothrombotic changes in patients with COVID-19 are associated with disease severity and mortality.Res Pract Thromb Haemost. 2020 Dec 6;5(1):132-141. doi: 10.1002/rth2.12462. eCollection 2021 Jan. Res Pract Thromb Haemost. 2020. PMID: 33537537 Free PMC article.
-
Dynamic Hemostasis and Fibrinolysis Assays in Intensive Care COVID-19 Patients and Association with Thrombosis and Bleeding-A Systematic Review and a Cohort Study.Semin Thromb Hemost. 2022 Feb;48(1):31-54. doi: 10.1055/s-0041-1735454. Epub 2021 Oct 29. Semin Thromb Hemost. 2022. PMID: 34715692
-
Prothrombotic disturbances of hemostasis of patients with severe COVID-19: A prospective longitudinal observational study.Thromb Res. 2021 Jan;197:20-23. doi: 10.1016/j.thromres.2020.10.025. Epub 2020 Oct 24. Thromb Res. 2021. PMID: 33161283 Free PMC article. No abstract available.
-
Global hemostatic profiling in patients with decompensated cirrhosis and bacterial infections.JHEP Rep. 2022 Apr 20;4(7):100493. doi: 10.1016/j.jhepr.2022.100493. eCollection 2022 Jul. JHEP Rep. 2022. PMID: 35647501 Free PMC article.
-
A new assay for global fibrinolysis capacity (GFC): Investigating a critical system regulating hemostasis and thrombosis and other extravascular functions.Transfus Apher Sci. 2018 Feb;57(1):118-126. doi: 10.1016/j.transci.2018.02.020. Epub 2018 Feb 20. Transfus Apher Sci. 2018. PMID: 29525568 Review.
Cited by
-
Monitoring of Anticoagulant Activity of Dabigatran and Rivaroxaban in the Presence of Heparins.J Clin Med. 2022 Apr 16;11(8):2236. doi: 10.3390/jcm11082236. J Clin Med. 2022. PMID: 35456329 Free PMC article.
-
MEDTEC Students against Coronavirus: Investigating the Role of Hemostatic Genes in the Predisposition to COVID-19 Severity.J Pers Med. 2021 Nov 9;11(11):1166. doi: 10.3390/jpm11111166. J Pers Med. 2021. PMID: 34834519 Free PMC article.
-
Comparison of Different Vascular Biomarkers for Predicting In-Hospital Mortality in Severe SARS-CoV-2 Infection.Microorganisms. 2024 Jan 22;12(1):229. doi: 10.3390/microorganisms12010229. Microorganisms. 2024. PMID: 38276214 Free PMC article.
-
Prevention of venous thromboembolism and haemostasis monitoring in patients with COVID-19: Updated proposals (April 2021): From the French working group on perioperative haemostasis (GIHP) and the French study group on thrombosis and haemostasis (GFHT), in collaboration with the French society of anaesthesia and intensive care (SFAR).Anaesth Crit Care Pain Med. 2021 Aug;40(4):100919. doi: 10.1016/j.accpm.2021.100919. Epub 2021 Jun 25. Anaesth Crit Care Pain Med. 2021. PMID: 34182166 Free PMC article. No abstract available.
-
Combining Heparin and a FX/Xa Aptamer to Reduce Thrombin Generation in Cardiopulmonary Bypass and COVID-19.Nucleic Acid Ther. 2022 Jun;32(3):139-150. doi: 10.1089/nat.2021.0077. Epub 2022 Jan 12. Nucleic Acid Ther. 2022. PMID: 35021888 Free PMC article.
References
-
- M. Hardy, I. Michaux, S. Lessire, J. Douxfils, J.-M. Dogné, M. Bareille, G. Horlait, P. Bulpa, C. Chapelle, S. Laporte, S. Testa, H. Jacqmin, T. Lecompte, A. Dive and F. Mullier. Prothrombotic hemostasis disturbances in patients with severe COVID-19: a prospective longitudinal observational cohort study. Thromb Res. 2020; 197:20-23. - PMC - PubMed
-
- Suzuki K, Wada H, Imai H, Iba T, Thachil J, Toh CH. A re-evaluation of the D-dimer cut-off value for making a diagnosis according to the ISTH overt-DIC diagnostic criteria: communication from the SSC of the ISTH. J. Thromb. Haemost. 2018;16(7):1442–1444. - PubMed
-
- Susen S, Tacquard CA, Godon A, Mansour A, Garrigue D, Nguyen P. Prevention of thrombotic risk in hospitalized patients with COVID19 and hemostasis monitoring: proposals from the French working group on perioperative haemostasis (GIHP) the French Sdy group on thrombosis and haemostasis (GFHT), in collaboration with the French Society for anaesthesia and intensive care (SFAR) Crit. Care. 2020;24:364. - PubMed
-
- Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Subcommittee on control of A. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J. Thromb. Haemost. 2015;13(11):2119–2126. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

