This is a preprint.
A Multi-Pronged Approach Targeting SARS-CoV-2 Proteins Using Ultra-Large Virtual Screening
- PMID: 33200116
- PMCID: PMC7668741
- DOI: 10.26434/chemrxiv.12682316
A Multi-Pronged Approach Targeting SARS-CoV-2 Proteins Using Ultra-Large Virtual Screening
Update in
-
A multi-pronged approach targeting SARS-CoV-2 proteins using ultra-large virtual screening.iScience. 2021 Feb 19;24(2):102021. doi: 10.1016/j.isci.2020.102021. Epub 2021 Jan 5. iScience. 2021. PMID: 33426509 Free PMC article.
Abstract
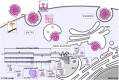
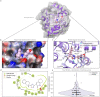
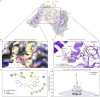
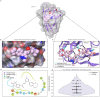
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), previously known as 2019 novel coronavirus (2019-nCoV), has spread rapidly across the globe, creating an unparalleled global health burden and spurring a deepening economic crisis. As of July 7th, 2020, almost seven months into the outbreak, there are no approved vaccines and few treatments available. Developing drugs that target multiple points in the viral life cycle could serve as a strategy to tackle the current as well as future coronavirus pandemics. Here we leverage the power of our recently developed in silico screening platform, VirtualFlow, to identify inhibitors that target SARS-CoV-2. VirtualFlow is able to efficiently harness the power of computing clusters and cloud-based computing platforms to carry out ultra-large scale virtual screens. In this unprecedented structure-based multi-target virtual screening campaign, we have used VirtualFlow to screen an average of approximately 1 billion molecules against each of 40 different target sites on 17 different potential viral and host targets in the cloud. In addition to targeting the active sites of viral enzymes, we also target critical auxiliary sites such as functionally important protein-protein interaction interfaces. This multi-target approach not only increases the likelihood of finding a potent inhibitor, but could also help identify a collection of anti-coronavirus drugs that would retain efficacy in the face of viral mutation. Drugs belonging to different regimen classes could be combined to develop possible combination therapies, and top hits that bind at highly conserved sites would be potential candidates for further development as coronavirus drugs. Here, we present the top 200 in silico hits for each target site. While in-house experimental validation of some of these compounds is currently underway, we want to make this array of potential inhibitor candidates available to researchers worldwide in consideration of the pressing need for fast-tracked drug development.
Keywords: ACE2; COVID-19 data; In silico; MPro; NSP16/NSP10; PLpro inhibitors; RdRP; SARS-CoV -2; SARS-CoV2 Small molecule inhibitor; Spike; TMPRSS2; computational biology; drug discovery; nsp10; nsp13; nsp14-exonuclease; nsp16; nsp3 macrdomain; nsp5; nsp7; nsp8; nsp9; nucleoprotein; virtual screening.
Conflict of interest statement
Alexander Chuprina, Dmytro Radchenko, and Iryna Iavniuk work for Enamine, Kyiv Ukraine. Igor Dziuba works for UkrOrgSyntez Ltd, Kyiv Ukraine. Olga Tarkhanova, Alla Plekhova, and Yurii Moroz work for Chemspace Kyiv, Ukraine. Enamine, UkrOrgSyntez, and Chemspace are companies that are involved in the synthesis and distribution of drug-like compounds. Yurii Moroz is a scientific advisor for Enamine.
Figures





















References
-
- Zhou Peng, Yang Xing-Lou, Wang Xian-Guang, Hu Ben, Zhang Lei, Zhang Wei, Si Hao-Rui, Zhu Yan, Li Bei, Huang Chao-Lin, Chen Hui-Dong, Chen Jing, Luo Yun, Guo Hua, Jiang Ren-Di, Liu Mei-Qin, Chen Ying, Shen Xu-Rui, Wang Xi, Zheng Xiao-Shuang, Zhao Kai, Chen Quan-Jiao, Deng Fei, Liu Lin-Lin, Yan Bing, Zhan Fa-Xian, Wang Yan-Yi, Xiao Geng-Fu, and Shi Zheng-Li. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798):270–273, 2020. ISSN 0028–0836 1476–4687. doi: 10.1038/s41586-020-2012-7 - DOI - PMC - PubMed
-
- Wu Fan, Zhao Su, Yu Bin, Chen Yan-Mei, Wang Wen, Song Zhi-Gang, Hu Yi, Tao Zhao-Wu, Tian Jun-Hua, Pei Yuan-Yuan, Yuan Ming-Li, Zhang Yu-Ling, Dai Fa-Hui, Liu Yi, Wang Qi-Min, Zheng Jiao-Jiao, Xu Lin, Holmes Edward C., and Zhang Yong-Zhen. A new coronavirus associated with human respiratory disease in China. Nature, 579(7798):265–269, 2020. ISSN 0028–0836 1476–4687. doi: 10.1038/s41586-020-2008-3 - DOI - PMC - PubMed
-
- Zhu Na, Zhang Dingyu, Wang Wenling, Li Xingwang, Yang Bo, Song Jingdong, Zhao Xiang, Huang Baoying, Shi Weifeng, Lu Roujian, Niu Peihua, Zhan Faxian, Ma Xuejun, Wang Dayan, Xu Wenbo, Wu Guizhen, Gao George F., and Tan Wenjie. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine, 382(8):727–733, 2020. ISSN 0028–4793 1533–4406. doi: 10.1056/NEJMoa2001017 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
