ACVR2B antagonism as a countermeasure to multi-organ perturbations in metastatic colorectal cancer cachexia
- PMID: 33200567
- PMCID: PMC7749603
- DOI: 10.1002/jcsm.12642
ACVR2B antagonism as a countermeasure to multi-organ perturbations in metastatic colorectal cancer cachexia
Abstract
Background: Advanced colorectal cancer (CRC) is often accompanied by the development of liver metastases, as well as cachexia, a multi-organ co-morbidity primarily affecting skeletal (SKM) and cardiac muscles. Activin receptor type 2B (ACVR2B) signalling is known to cause SKM wasting, and its inhibition restores SKM mass and prolongs survival in cancer. Using a recently generated mouse model, here we tested whether ACVR2B blockade could preserve multiple organs, including skeletal and cardiac muscle, in the presence of metastatic CRC.
Methods: NSG male mice (8 weeks old) were injected intrasplenically with HCT116 human CRC cells (mHCT116), while sham-operated animals received saline (n = 5-10 per group). Sham and tumour-bearing mice received weekly injections of ACVR2B/Fc, a synthetic peptide inhibitor of ACVR2B.
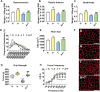
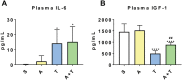
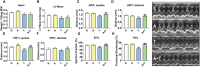
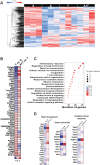
Results: mHCT116 hosts displayed losses in fat mass ( - 79%, P < 0.0001), bone mass ( - 39%, P < 0.05), and SKM mass (quadriceps: - 22%, P < 0.001), in line with reduced muscle cross-sectional area ( - 24%, P < 0.01) and plantarflexion force ( - 28%, P < 0.05). Further, despite only moderately affected heart size, cardiac function was significantly impaired (ejection fraction %: - 16%, P < 0.0001; fractional shortening %: - 25%, P < 0.0001) in the mHCT116 hosts. Conversely, ACVR2B/Fc preserved fat mass ( + 238%, P < 0.001), bone mass ( + 124%, P < 0.0001), SKM mass (quadriceps: + 31%, P < 0.0001), size (cross-sectional area: + 43%, P < 0.0001) and plantarflexion force ( + 28%, P < 0.05) in tumour hosts. Cardiac function was also completely preserved in tumour hosts receiving ACVR2B/Fc (ejection fraction %: + 19%, P < 0.0001), despite no effect on heart size. RNA sequencing analysis of heart muscle revealed rescue of genes related to cardiac development and contraction in tumour hosts treated with ACVR2B/Fc.
Conclusions: Our metastatic CRC model recapitulates the multi-systemic derangements of cachexia by displaying loss of fat, bone, and SKM along with decreased muscle strength in mHCT116 hosts. Additionally, with evidence of severe cardiac dysfunction, our data support the development of cardiac cachexia in the occurrence of metastatic CRC. Notably, ACVR2B antagonism preserved adipose tissue, bone, and SKM, whereas muscle and cardiac functions were completely maintained upon treatment. Altogether, our observations implicate ACVR2B signalling in the development of multi-organ perturbations in metastatic CRC and further dictate that ACVR2B represents a promising therapeutic target to preserve body composition and functionality in cancer cachexia.
Keywords: Activin signalling; Bone; Cachexia; Colorectal cancer; Heart; Liver metastases; Skeletal muscle.
© 2020 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures




Similar articles
-
HCT116 colorectal liver metastases exacerbate muscle wasting in a mouse model for the study of colorectal cancer cachexia.Dis Model Mech. 2020 Jan 24;13(1):dmm043166. doi: 10.1242/dmm.043166. Dis Model Mech. 2020. PMID: 31915140 Free PMC article.
-
MC38 Tumors Induce Musculoskeletal Defects in Colorectal Cancer.Int J Mol Sci. 2021 Feb 2;22(3):1486. doi: 10.3390/ijms22031486. Int J Mol Sci. 2021. PMID: 33540821 Free PMC article.
-
The systemic activin response to pancreatic cancer: implications for effective cancer cachexia therapy.J Cachexia Sarcopenia Muscle. 2019 Oct;10(5):1083-1101. doi: 10.1002/jcsm.12461. Epub 2019 Jul 8. J Cachexia Sarcopenia Muscle. 2019. PMID: 31286691 Free PMC article.
-
STAT3 in the systemic inflammation of cancer cachexia.Semin Cell Dev Biol. 2016 Jun;54:28-41. doi: 10.1016/j.semcdb.2016.02.009. Epub 2016 Feb 6. Semin Cell Dev Biol. 2016. PMID: 26860754 Free PMC article. Review.
-
The Role of Tumor Microenvironment Cells in Colorectal Cancer (CRC) Cachexia.Int J Mol Sci. 2021 Feb 4;22(4):1565. doi: 10.3390/ijms22041565. Int J Mol Sci. 2021. PMID: 33557173 Free PMC article. Review.
Cited by
-
GDF8 inhibition enhances musculoskeletal recovery and mitigates posttraumatic osteoarthritis following joint injury.Sci Adv. 2023 Dec;9(48):eadi9134. doi: 10.1126/sciadv.adi9134. Epub 2023 Nov 29. Sci Adv. 2023. PMID: 38019905 Free PMC article.
-
The Mitochondria-Targeting Agent MitoQ Improves Muscle Atrophy, Weakness and Oxidative Metabolism in C26 Tumor-Bearing Mice.Front Cell Dev Biol. 2022 Mar 22;10:861622. doi: 10.3389/fcell.2022.861622. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35392166 Free PMC article.
-
Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) R47H Variant Causes Distinct Age- and Sex-Dependent Musculoskeletal Alterations in Mice.J Bone Miner Res. 2022 Jul;37(7):1366-1381. doi: 10.1002/jbmr.4572. Epub 2022 Jun 7. J Bone Miner Res. 2022. PMID: 35575023 Free PMC article.
-
Acetyl-Coenzyme A Synthetase 2 Potentiates Macropinocytosis and Muscle Wasting Through Metabolic Reprogramming in Pancreatic Cancer.Gastroenterology. 2022 Nov;163(5):1281-1293.e1. doi: 10.1053/j.gastro.2022.06.058. Epub 2022 Jun 28. Gastroenterology. 2022. PMID: 35777482 Free PMC article.
-
Skeletal Muscle Deconditioning in Breast Cancer Patients Undergoing Chemotherapy: Current Knowledge and Insights From Other Cancers.Front Cell Dev Biol. 2021 Sep 14;9:719643. doi: 10.3389/fcell.2021.719643. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34595171 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. - PubMed
-
- Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer 2002;38:1023–1033. - PubMed
-
- Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ. Cancer cachexia: beyond weight loss. J Oncol Pract 2016;12:1163–1171. - PubMed
-
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–166. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

