Prediction of optimal contrast times post-imaging agent administration to inform personalized fluorescence-guided surgery
- PMID: 33200596
- PMCID: PMC7667427
- DOI: 10.1117/1.JBO.25.11.116005
Prediction of optimal contrast times post-imaging agent administration to inform personalized fluorescence-guided surgery
Abstract
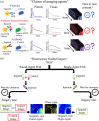
Significance: Fluorescence guidance in cancer surgery (FGS) using molecular-targeted contrast agents is accelerating, yet the influence of individual patients' physiology on the optimal time to perform surgery post-agent-injection is not fully understood.
Aim: Develop a mathematical framework and analytical expressions to estimate patient-specific time-to-maximum contrast after imaging agent administration for single- and paired-agent (coadministration of targeted and control agents) protocols.
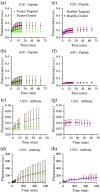
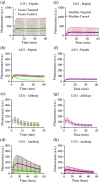
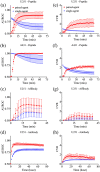
Approach: The framework was validated in mouse subcutaneous xenograft studies for three classes of imaging agents: peptide, antibody mimetic, and antibody. Analytical expressions estimating time-to-maximum-tumor-discrimination potential were evaluated over a range of parameters using the validated framework for human cancer parameters.
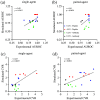
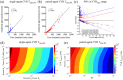
Results: Correlations were observed between simulations and matched experiments and metrics of tumor discrimination potential (p < 0.05). Based on human cancer physiology, times-to-maximum contrast for peptide and antibody mimetic agents were <200 min, >15 h for antibodies, on average. The analytical estimates of time-to-maximum tumor discrimination performance exhibited errors of <10 % on average, whereas patient-to-patient variance is expected to be greater than 100%.
Conclusion: We demonstrated that analytical estimates of time-to-maximum contrast in FGS carried out patient-to-patient can outperform the population average time-to-maximum contrast used currently in clinical trials. Such estimates can be made with preoperative DCE-MRI (or similar) and knowledge of the targeted agent's binding affinity.
Keywords: fluorescence-guided surgery; intraoperative visualization; kinetic modeling; optimal time of surgery; paired-agent imaging.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

