Design, Synthesis, and Biological Evaluation of Chemically and Biologically Diverse Pyrroquinoline Pseudo Natural Products
- PMID: 33200868
- PMCID: PMC7986669
- DOI: 10.1002/anie.202013731
Design, Synthesis, and Biological Evaluation of Chemically and Biologically Diverse Pyrroquinoline Pseudo Natural Products
Abstract
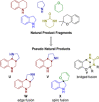
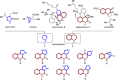
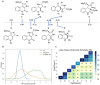
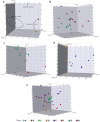
Natural product (NP) structures are a rich source of inspiration for the discovery of new biologically relevant chemical matter. In natural product inspired pseudo-NPs, NP-derived fragments are combined de novo in unprecedented arrangements. Described here is the design and synthesis of a 155-member pyrroquinoline pseudo-NP collection in which fragments characteristic of the tetrahydroquinoline and pyrrolidine NP classes are combined with eight different connectivities and regioisomeric arrangements. Cheminformatic analysis and biological evaluation of the compound collection by means of phenotyping in the morphological "cell painting" assay followed by principal component analysis revealed that the pseudo-NP classes are chemically diverse and that bioactivity patterns differ markedly, and are dependent on connectivity and regioisomeric arrangement of the fragments.
Keywords: cell painting; cheminformatics; cycloaddition; heterocycles; natural products.
© 2020 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Natural product fragment combination to performance-diverse pseudo-natural products.Nat Commun. 2021 Mar 25;12(1):1883. doi: 10.1038/s41467-021-22174-4. Nat Commun. 2021. PMID: 33767198 Free PMC article.
-
Synthesis of Indofulvin Pseudo-Natural Products Yields a New Autophagy Inhibitor Chemotype.Adv Sci (Weinh). 2021 Oct;8(19):e2102042. doi: 10.1002/advs.202102042. Epub 2021 Aug 4. Adv Sci (Weinh). 2021. PMID: 34346568 Free PMC article.
-
Combination of Pseudo-Natural Product Design and Formal Natural Product Ring Distortion Yields Stereochemically and Biologically Diverse Pseudo-Sesquiterpenoid Alkaloids.Angew Chem Int Ed Engl. 2021 Sep 20;60(39):21384-21395. doi: 10.1002/anie.202106654. Epub 2021 Aug 19. Angew Chem Int Ed Engl. 2021. PMID: 34297473 Free PMC article.
-
Principle and design of pseudo-natural products.Nat Chem. 2020 Mar;12(3):227-235. doi: 10.1038/s41557-019-0411-x. Epub 2020 Feb 3. Nat Chem. 2020. PMID: 32015480 Review.
-
Guided by evolution: from biology oriented synthesis to pseudo natural products.Nat Prod Rep. 2020 Nov 18;37(11):1497-1510. doi: 10.1039/d0np00015a. Nat Prod Rep. 2020. PMID: 33020792 Review.
Cited by
-
Pseudo Natural Products-Chemical Evolution of Natural Product Structure.Angew Chem Int Ed Engl. 2021 Jul 12;60(29):15705-15723. doi: 10.1002/anie.202016575. Epub 2021 Mar 23. Angew Chem Int Ed Engl. 2021. PMID: 33644925 Free PMC article. Review.
-
Pseudonatural Products Occur Frequently in Biologically Relevant Compounds.J Chem Inf Model. 2021 Nov 22;61(11):5458-5468. doi: 10.1021/acs.jcim.1c01084. Epub 2021 Oct 20. J Chem Inf Model. 2021. PMID: 34669418 Free PMC article.
-
A divergent intermediate strategy yields biologically diverse pseudo-natural products.Nat Chem. 2024 Jun;16(6):945-958. doi: 10.1038/s41557-024-01458-4. Epub 2024 Feb 16. Nat Chem. 2024. PMID: 38365941 Free PMC article.
-
Cell Painting: a decade of discovery and innovation in cellular imaging.Nat Methods. 2025 Feb;22(2):254-268. doi: 10.1038/s41592-024-02528-8. Epub 2024 Dec 5. Nat Methods. 2025. PMID: 39639168 Free PMC article. Review.
-
Chemical Evolution of Natural Product Structure.J Am Chem Soc. 2022 Mar 2;144(8):3314-3329. doi: 10.1021/jacs.1c11270. Epub 2022 Feb 21. J Am Chem Soc. 2022. PMID: 35188375 Free PMC article.
References
-
- Eder J., Sedrani R., Wiesmann C., Nat. Rev. Drug Discovery 2014, 13, 577–587. - PubMed
-
- Wetzel S., Bon R. S., Kumar K., Waldmann H., Angew. Chem. Int. Ed. 2011, 50, 10800–10826; - PubMed
- Angew. Chem. 2011, 123, 10990–11018.
-
- Rafferty R. J., Hicklin R. W., Maloof K. A., Hergenrother P. J., Angew. Chem. Int. Ed. 2014, 53, 220–224; - PubMed
- Angew. Chem. 2014, 126, 224–228.
-
- Karageorgis G., Foley D. J., Laraia L., Waldmann H., Nat. Chem. 2020, 12, 227–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

