Decarboxylative Halogenation of Organic Compounds
- PMID: 33200917
- PMCID: PMC7884003
- DOI: 10.1021/acs.chemrev.0c00813
Decarboxylative Halogenation of Organic Compounds
Abstract
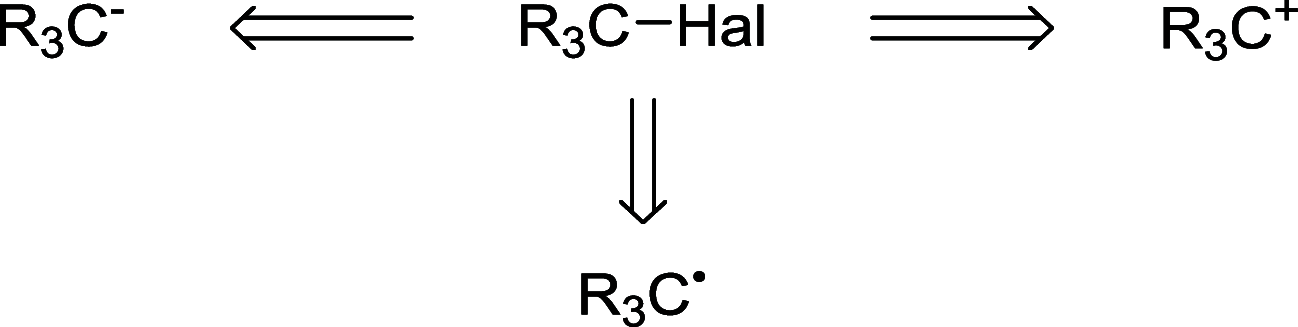
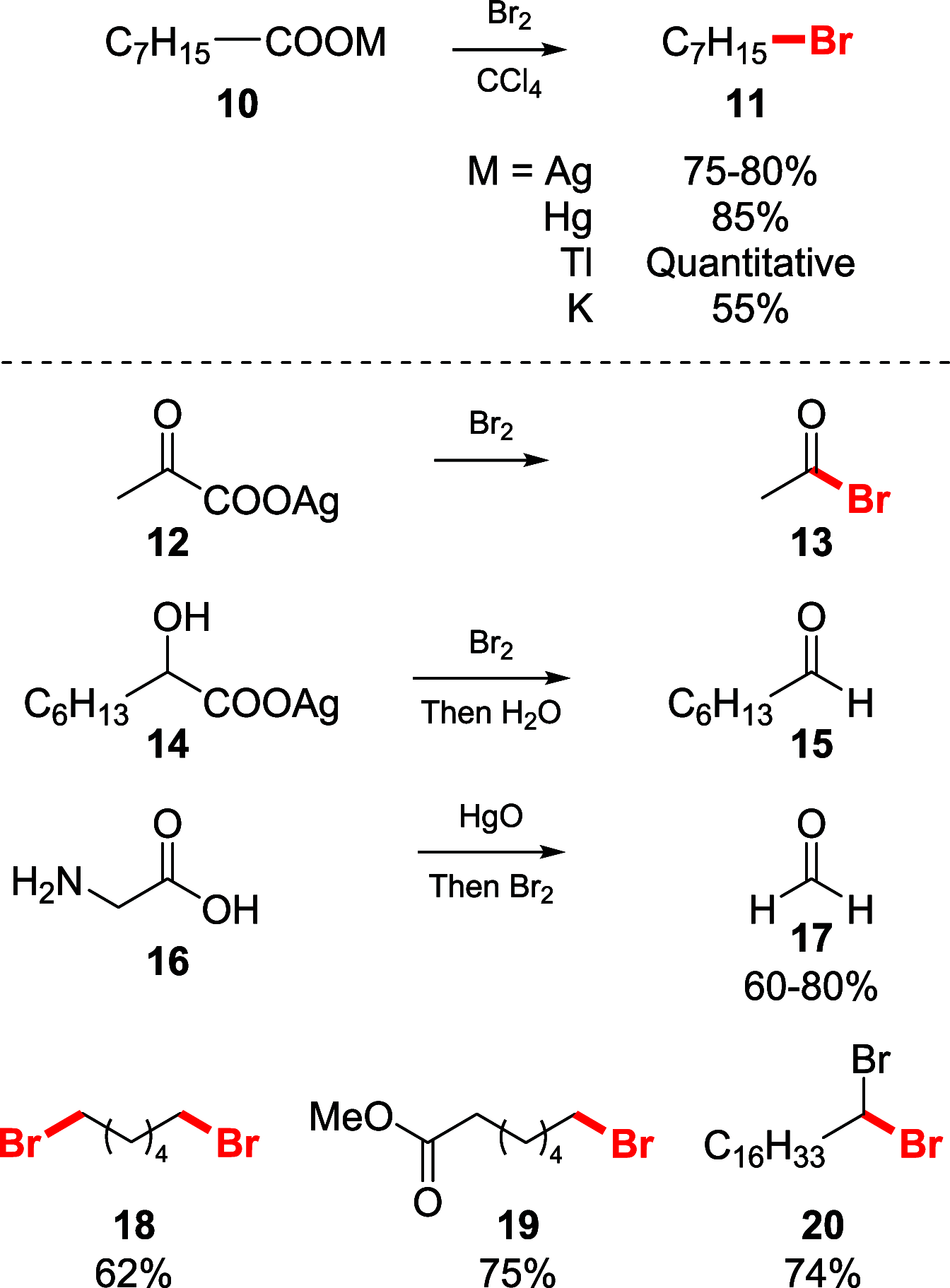
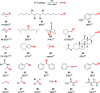
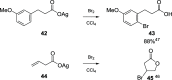
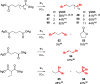
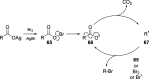
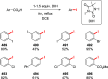
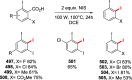
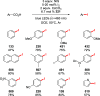
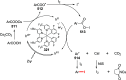
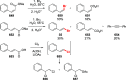
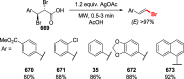
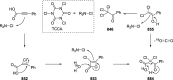
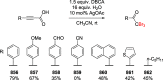
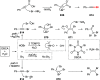
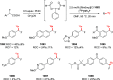
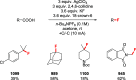
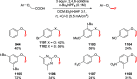
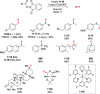
Decarboxylative halogenation, or halodecarboxylation, represents one of the fundamental key methods for the synthesis of ubiquitous organic halides. The method is based on conversion of carboxylic acids to the corresponding organic halides via selective cleavage of a carbon-carbon bond between the skeleton of the molecule and the carboxylic group and the liberation of carbon dioxide. In this review, we discuss and analyze major approaches for the conversion of alkanoic, alkenoic, acetylenic, and (hetero)aromatic acids to the corresponding alkyl, alkenyl, alkynyl, and (hetero)aryl halides. These methods include the preparation of families of valuable organic iodides, bromides, chlorides, and fluorides. The historic and modern methods for halodecarboxylation reactions are broadly discussed, including analysis of their advantages and drawbacks. We critically address the features, reaction selectivity, substrate scopes, and limitations of the approaches. In the available cases, mechanistic details of the reactions are presented, and the generality and uniqueness of the different mechanistic pathways are highlighted. The challenges, opportunities, and future directions in the field of decarboxylative halogenation are provided.
Conflict of interest statement
The authors declare no competing financial interest.
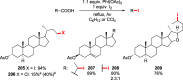
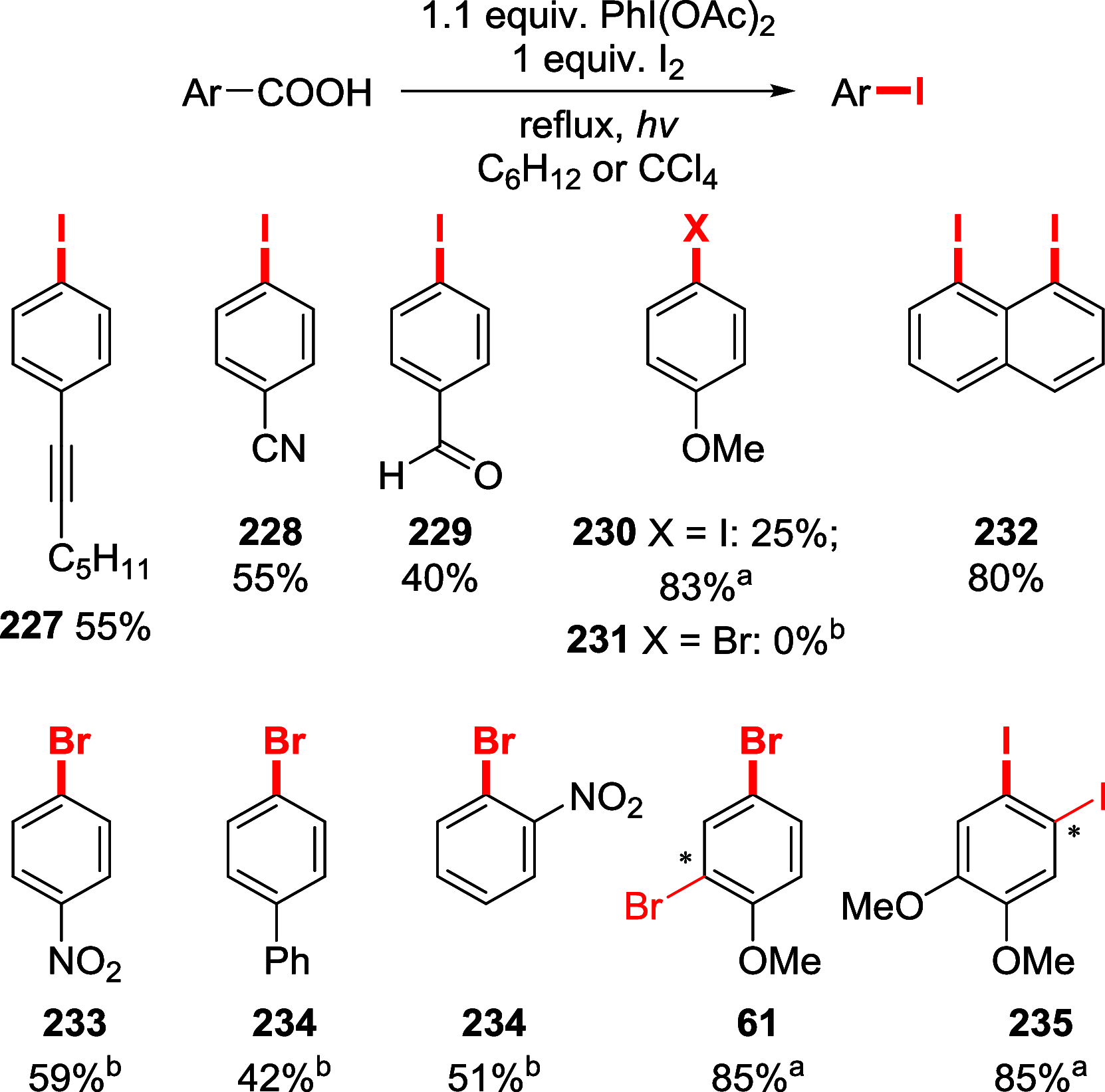
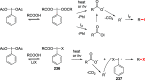
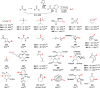
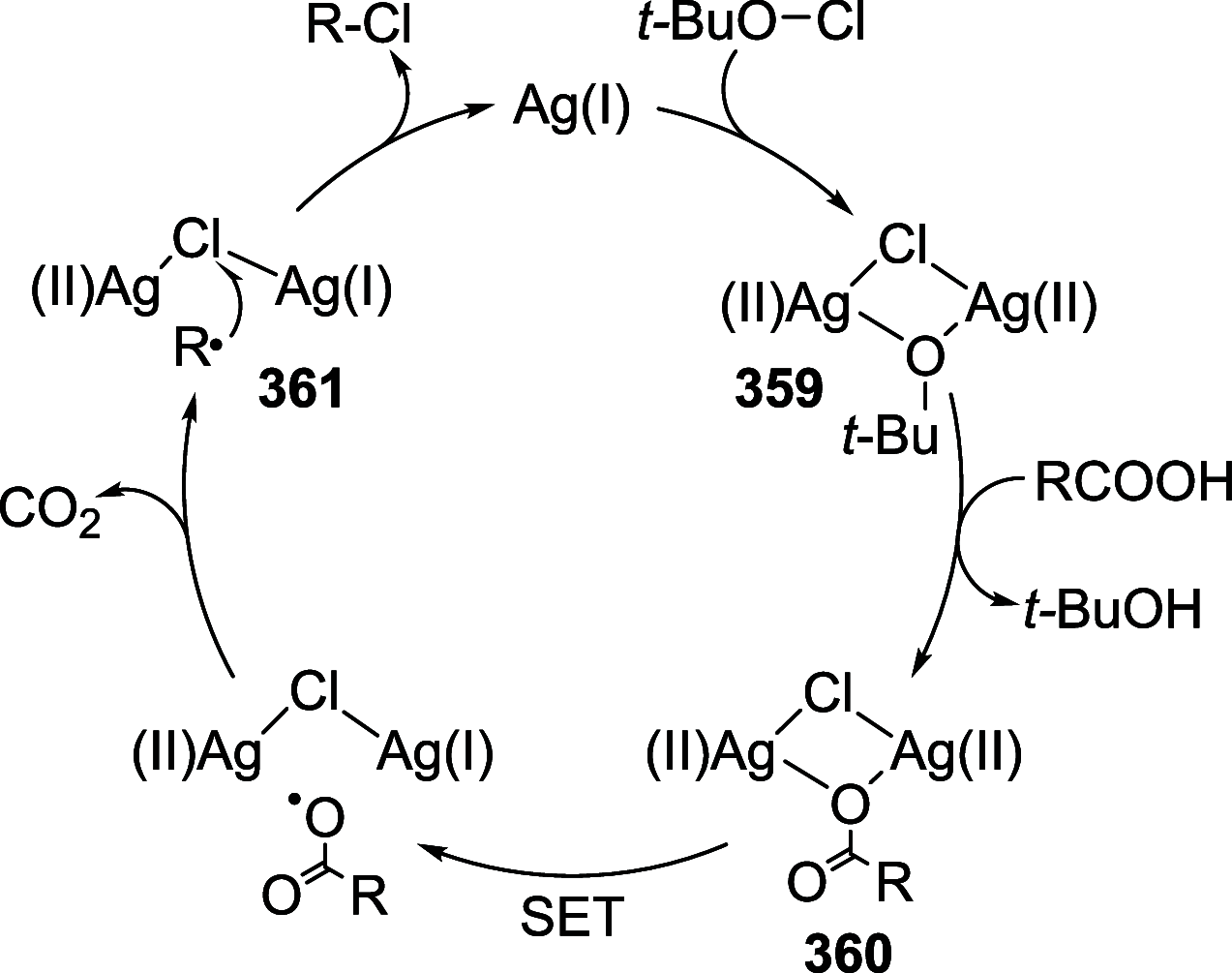
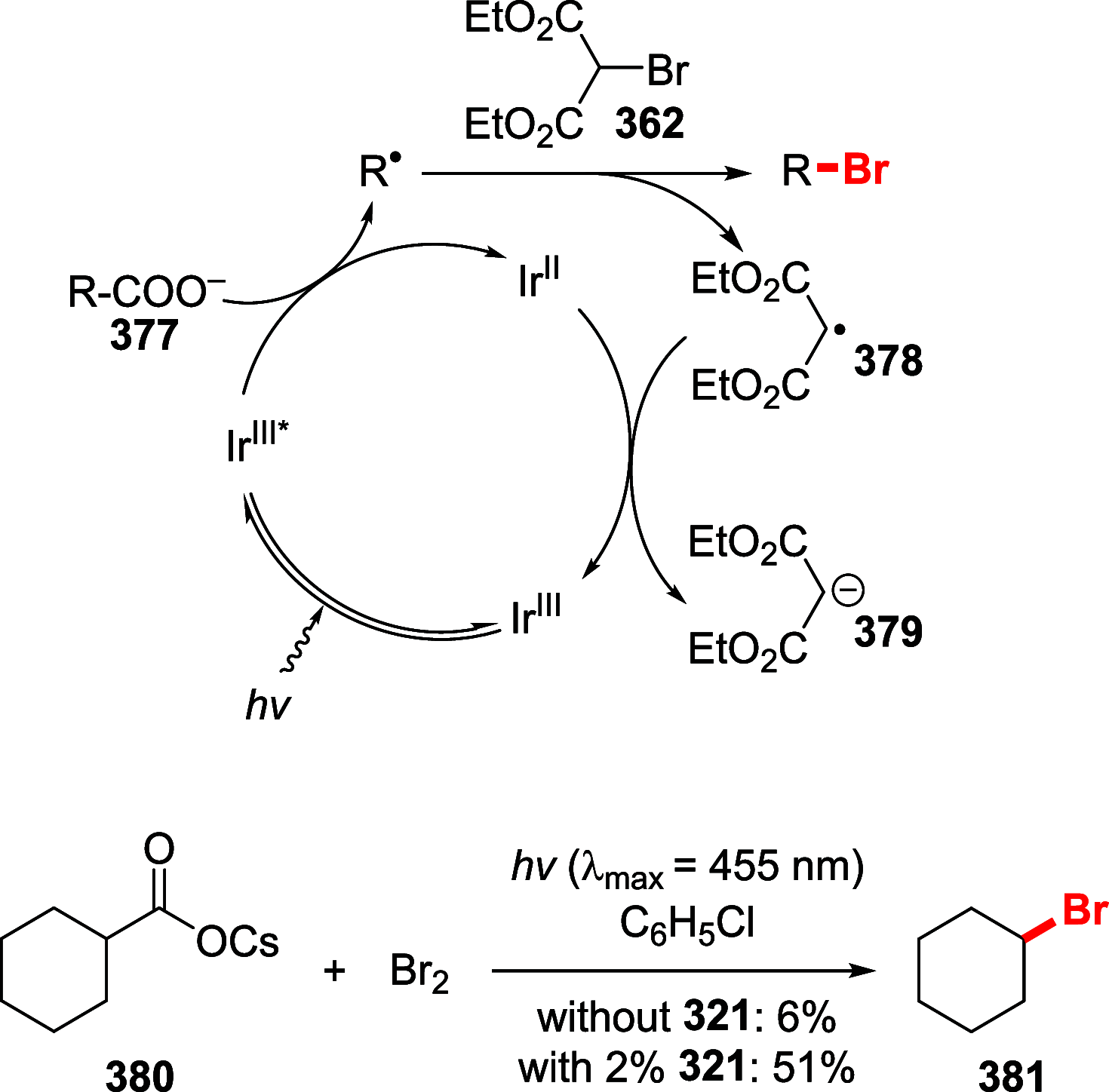
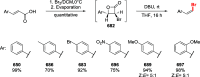
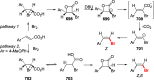
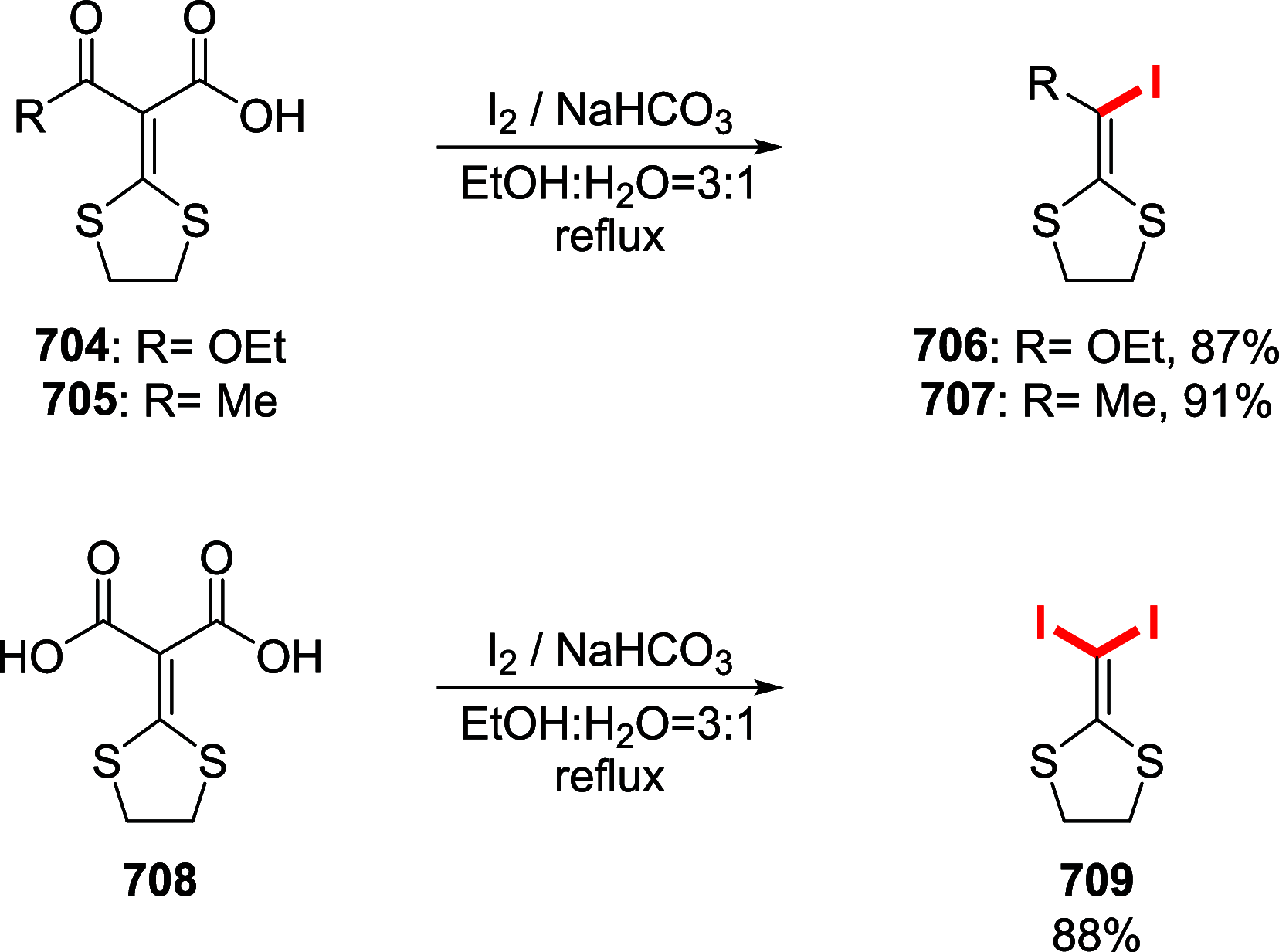
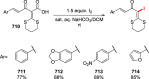
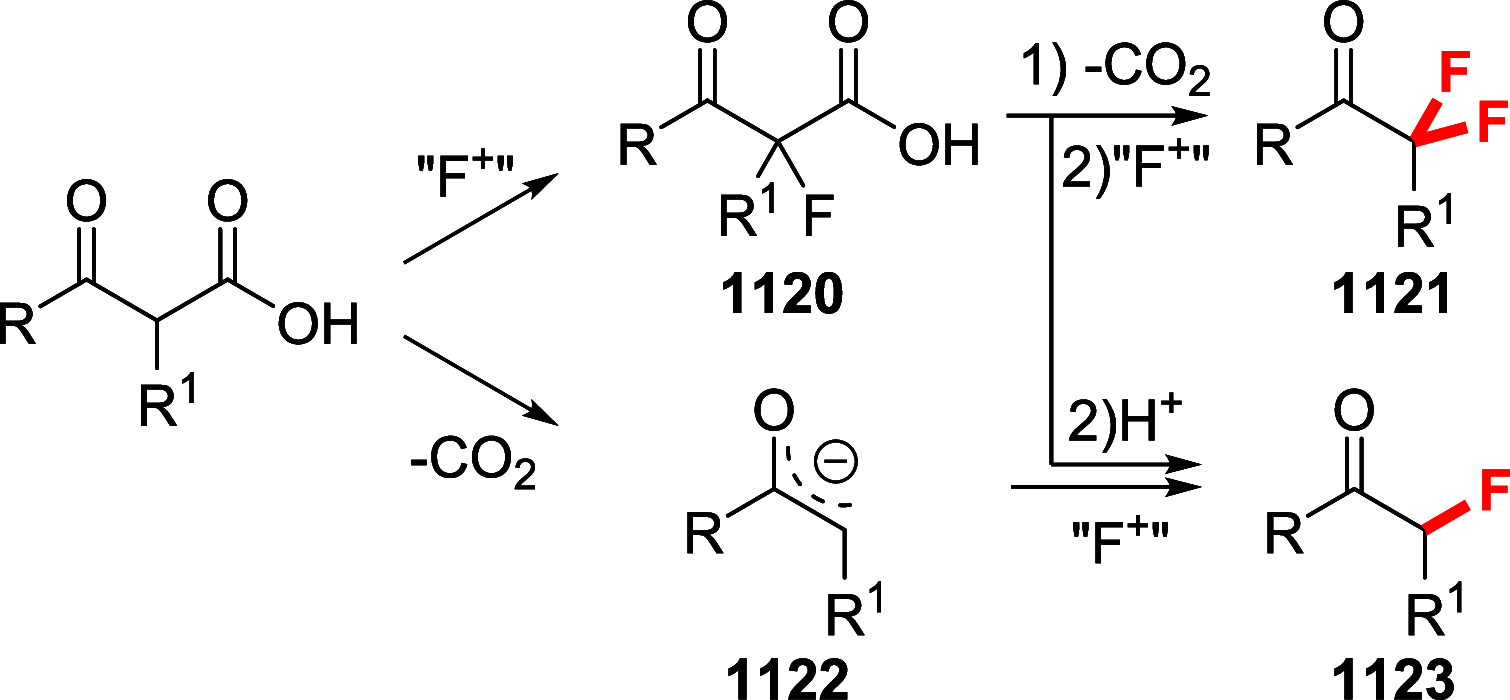
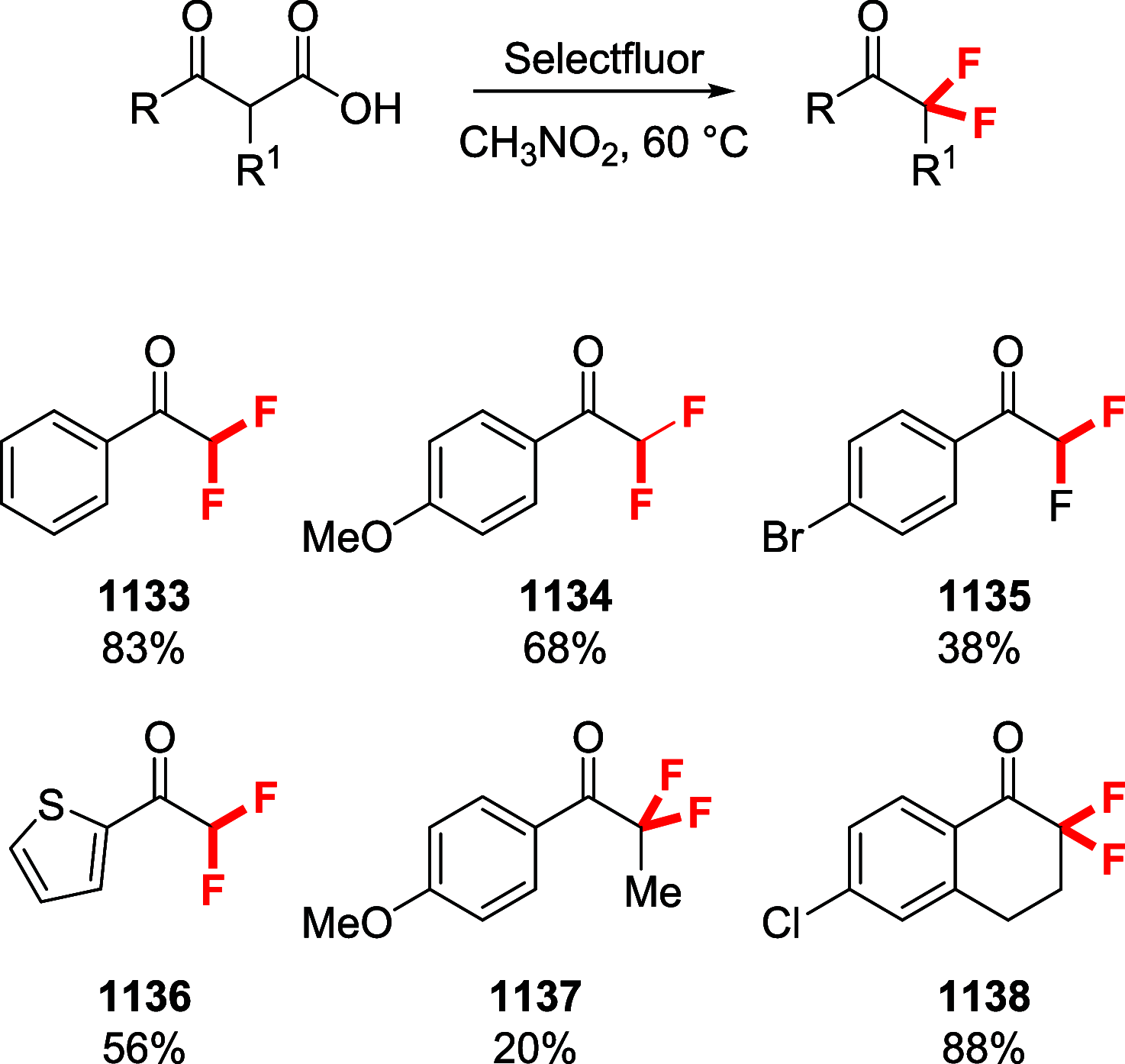
Figures




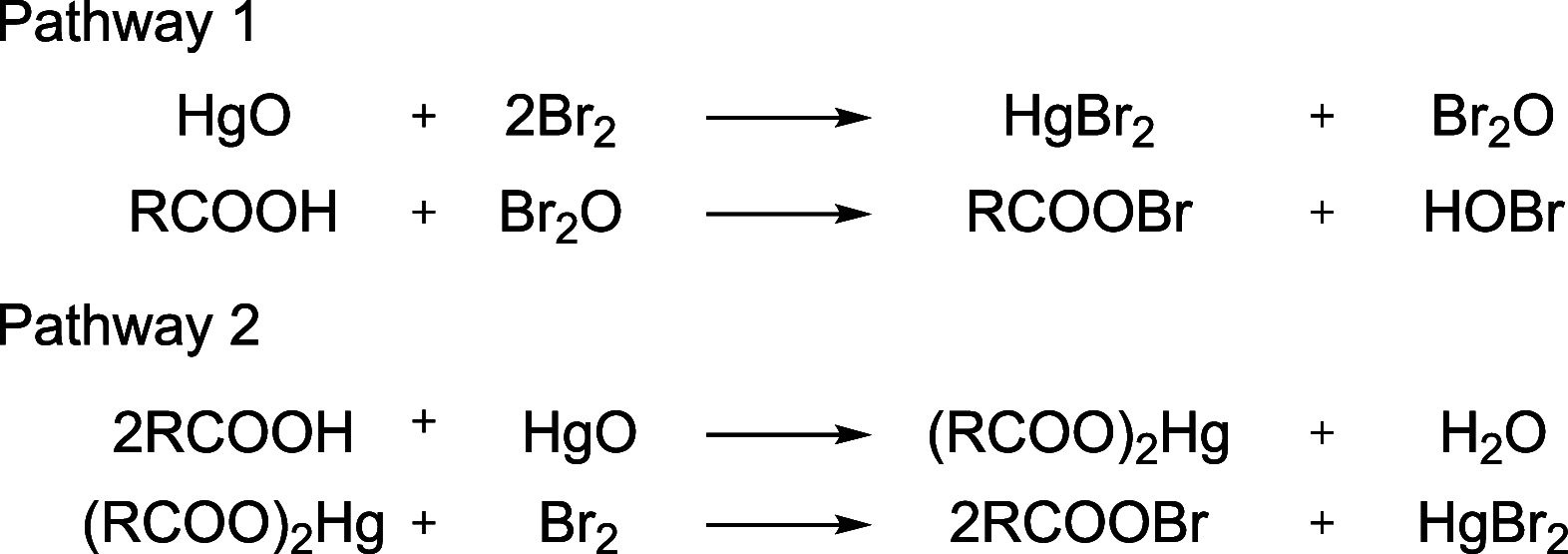
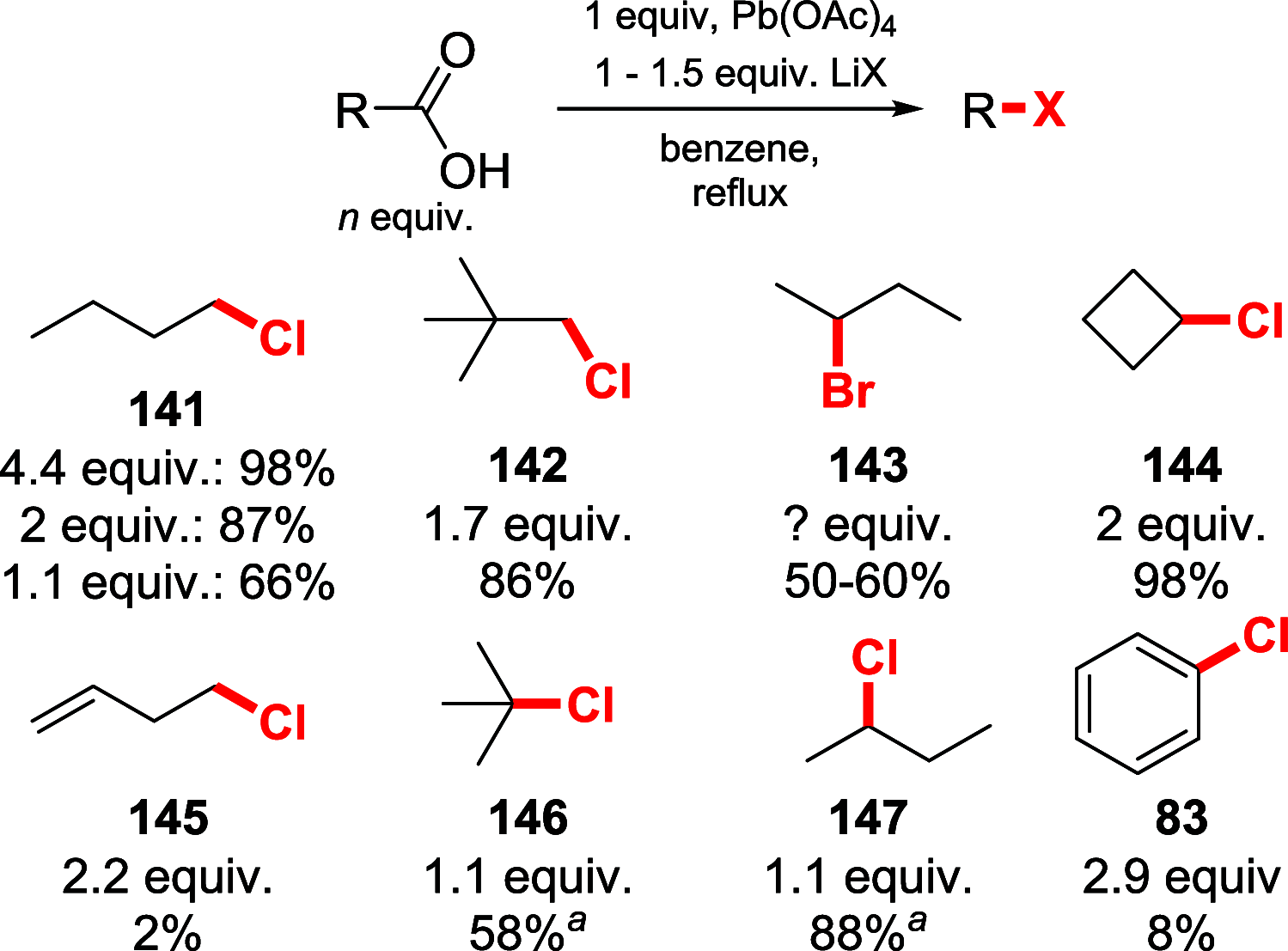
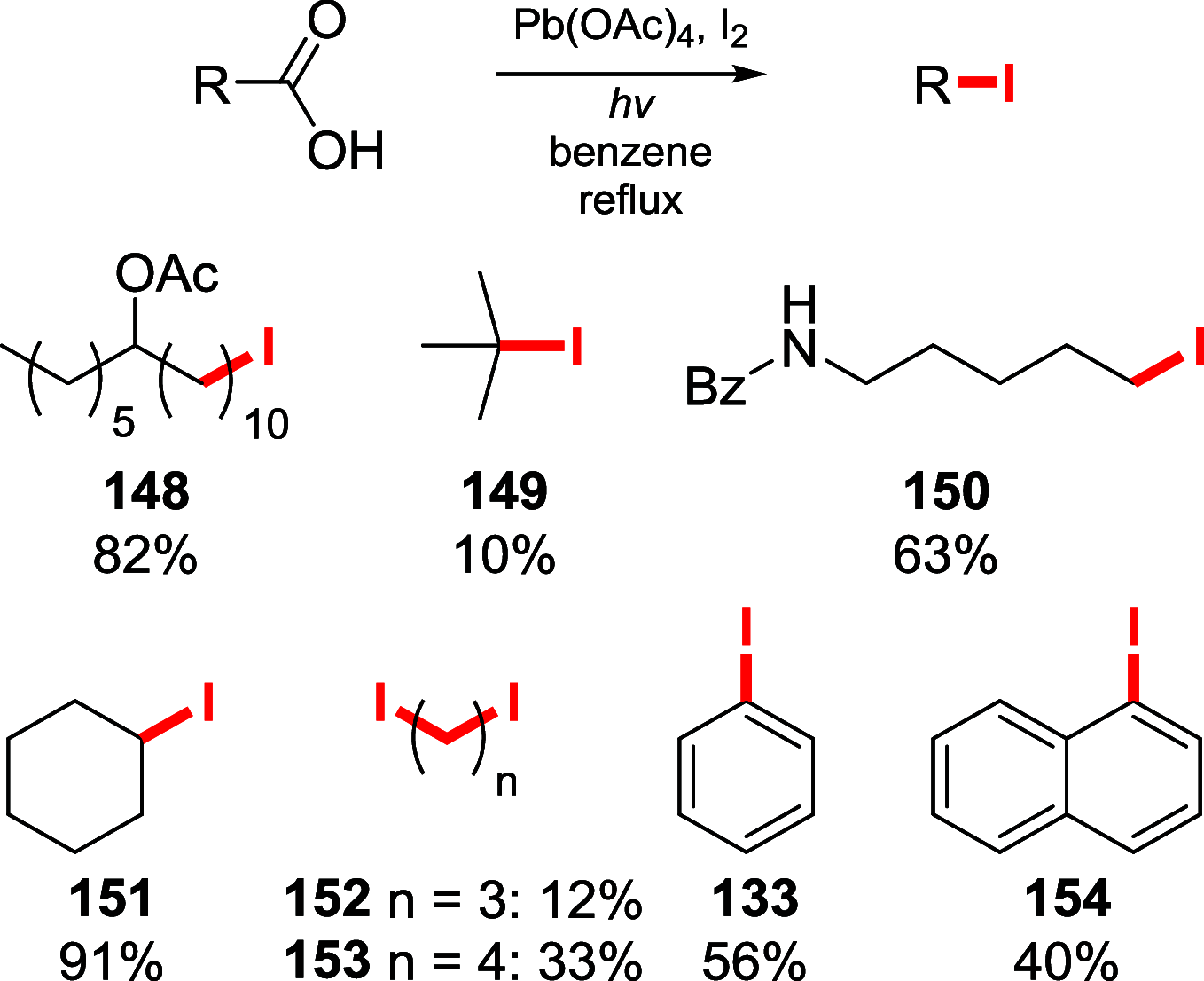
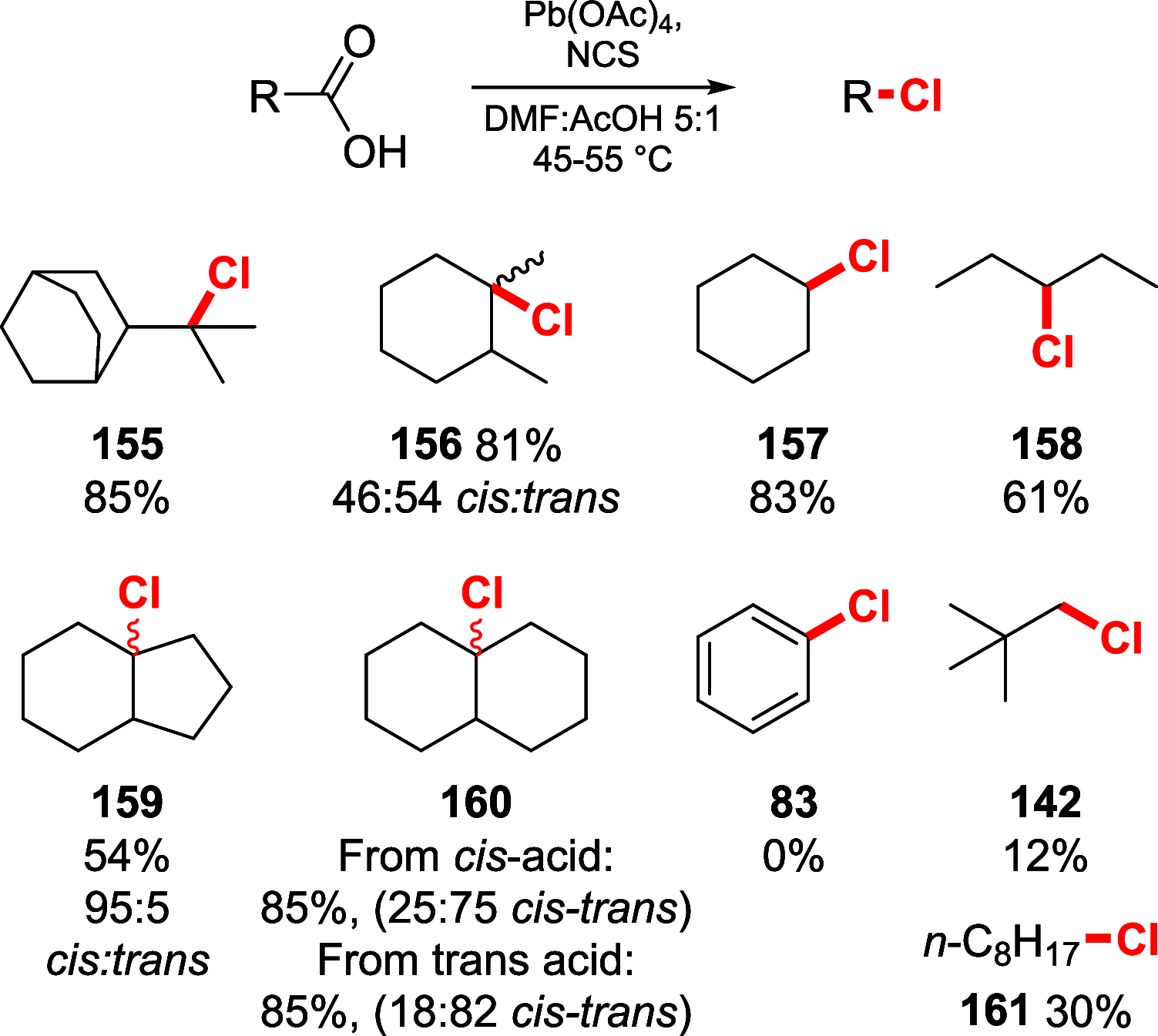



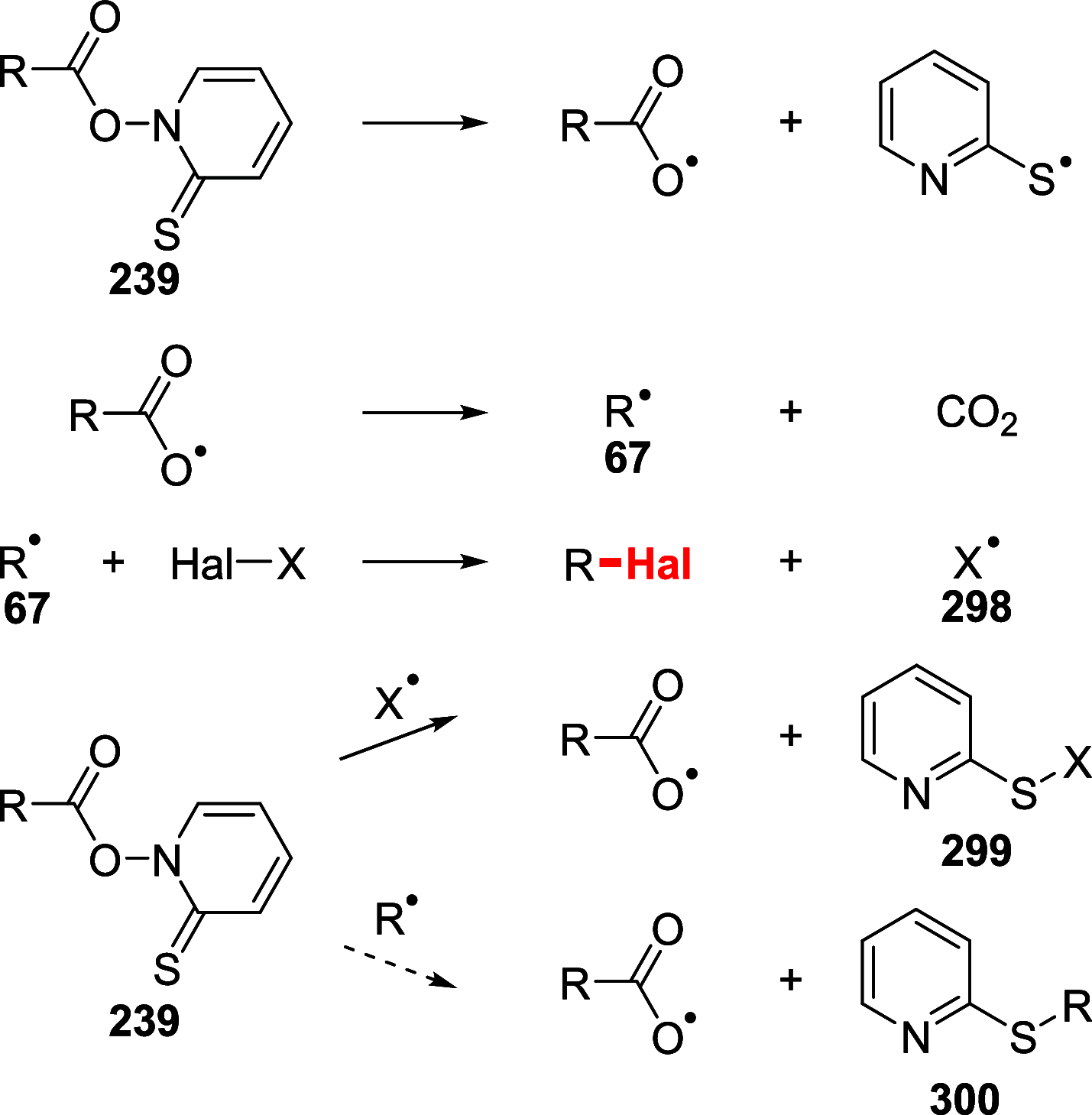
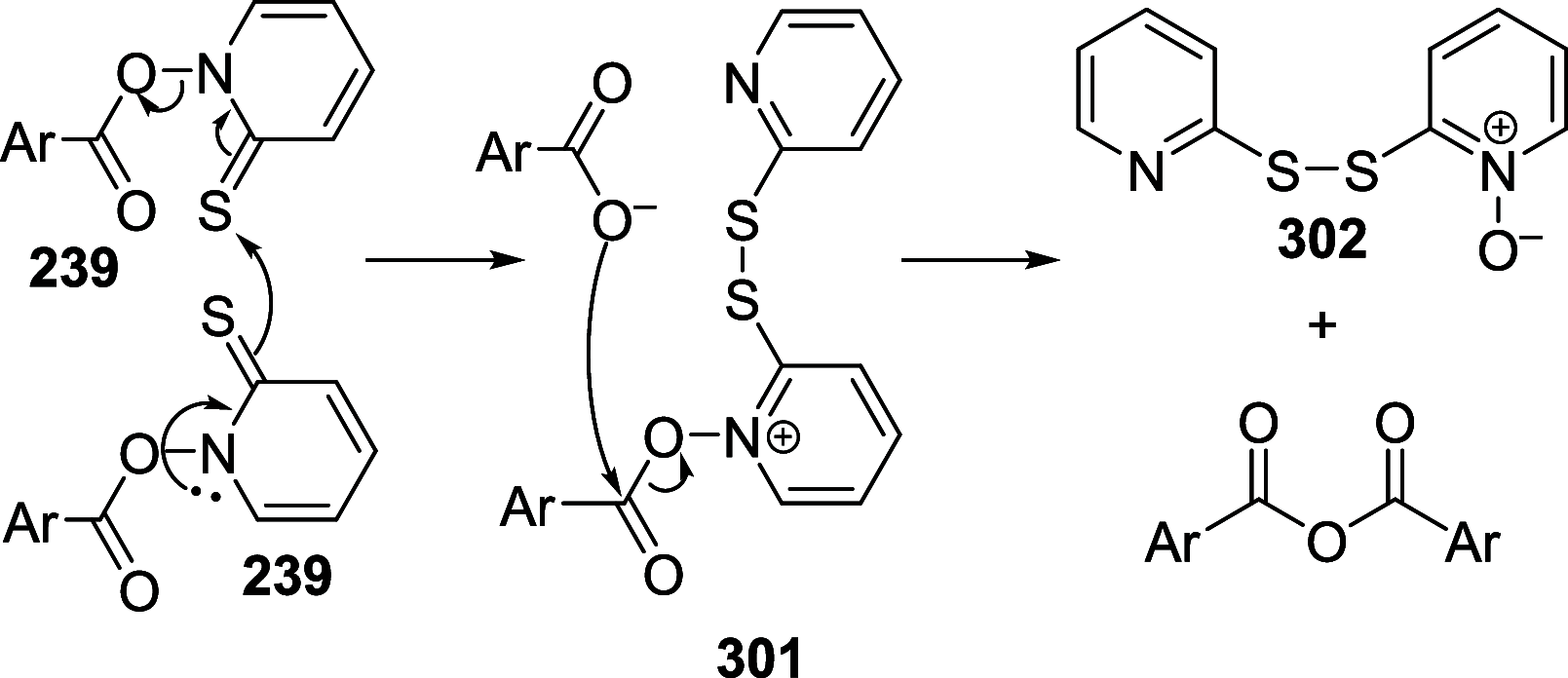

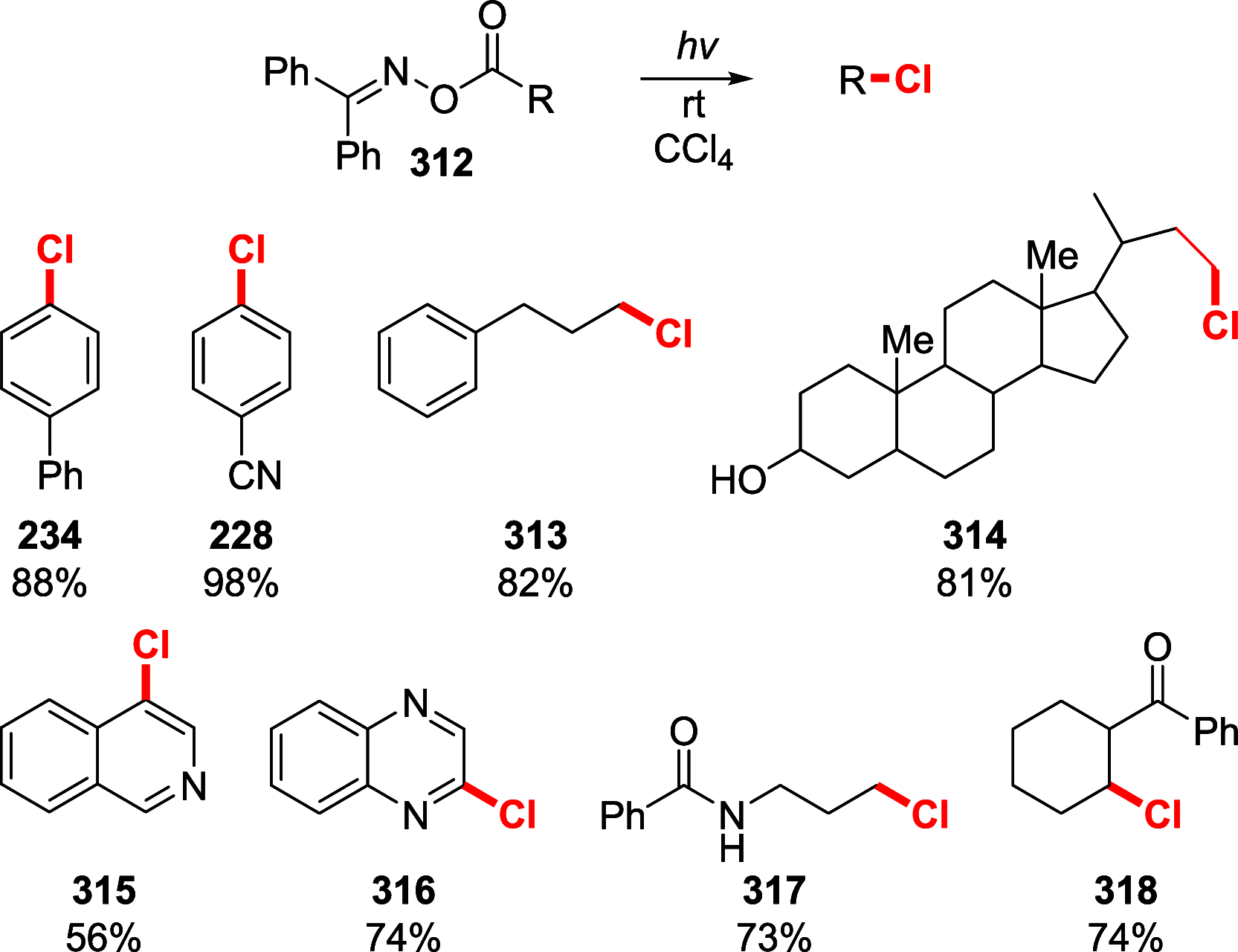

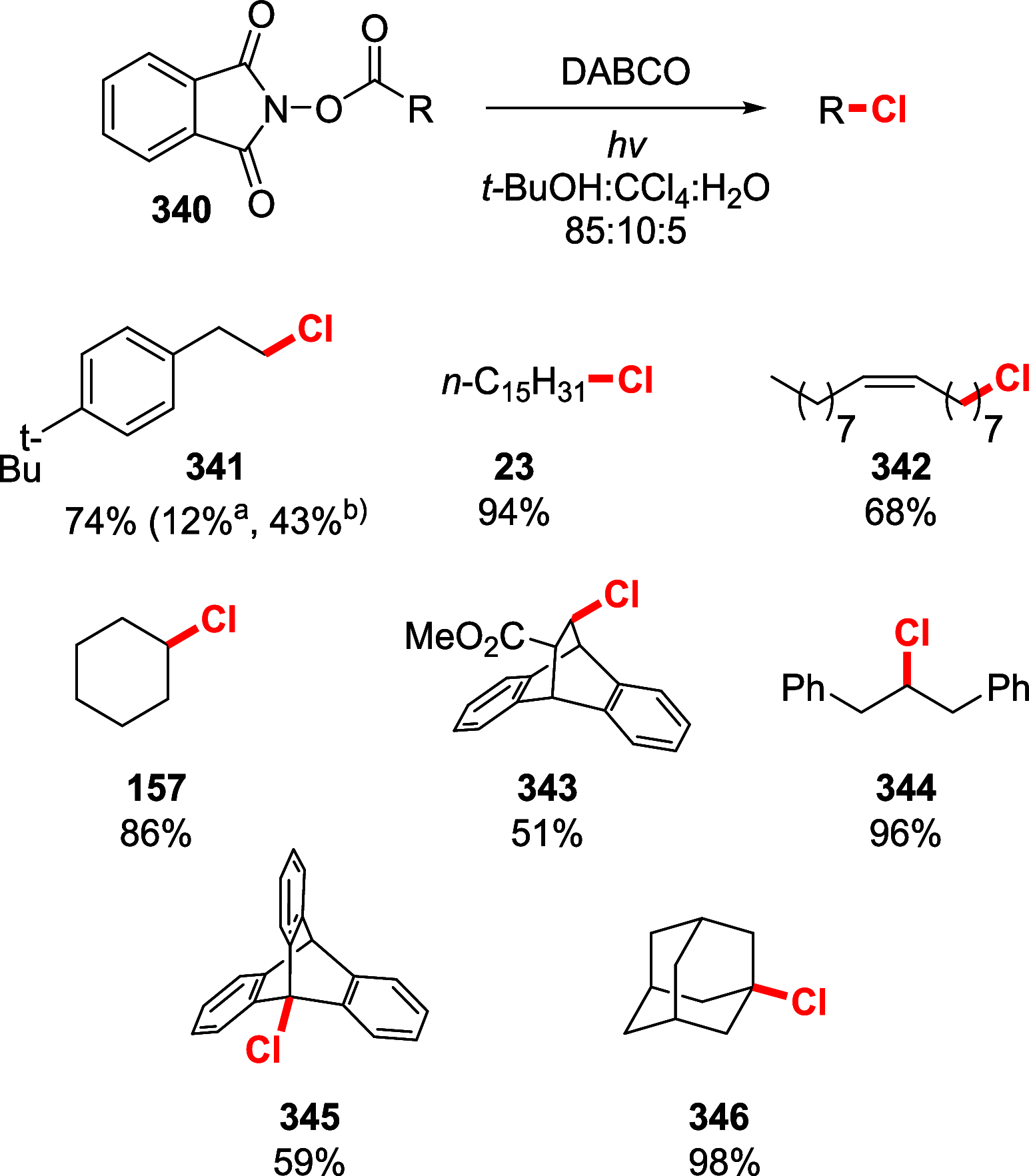




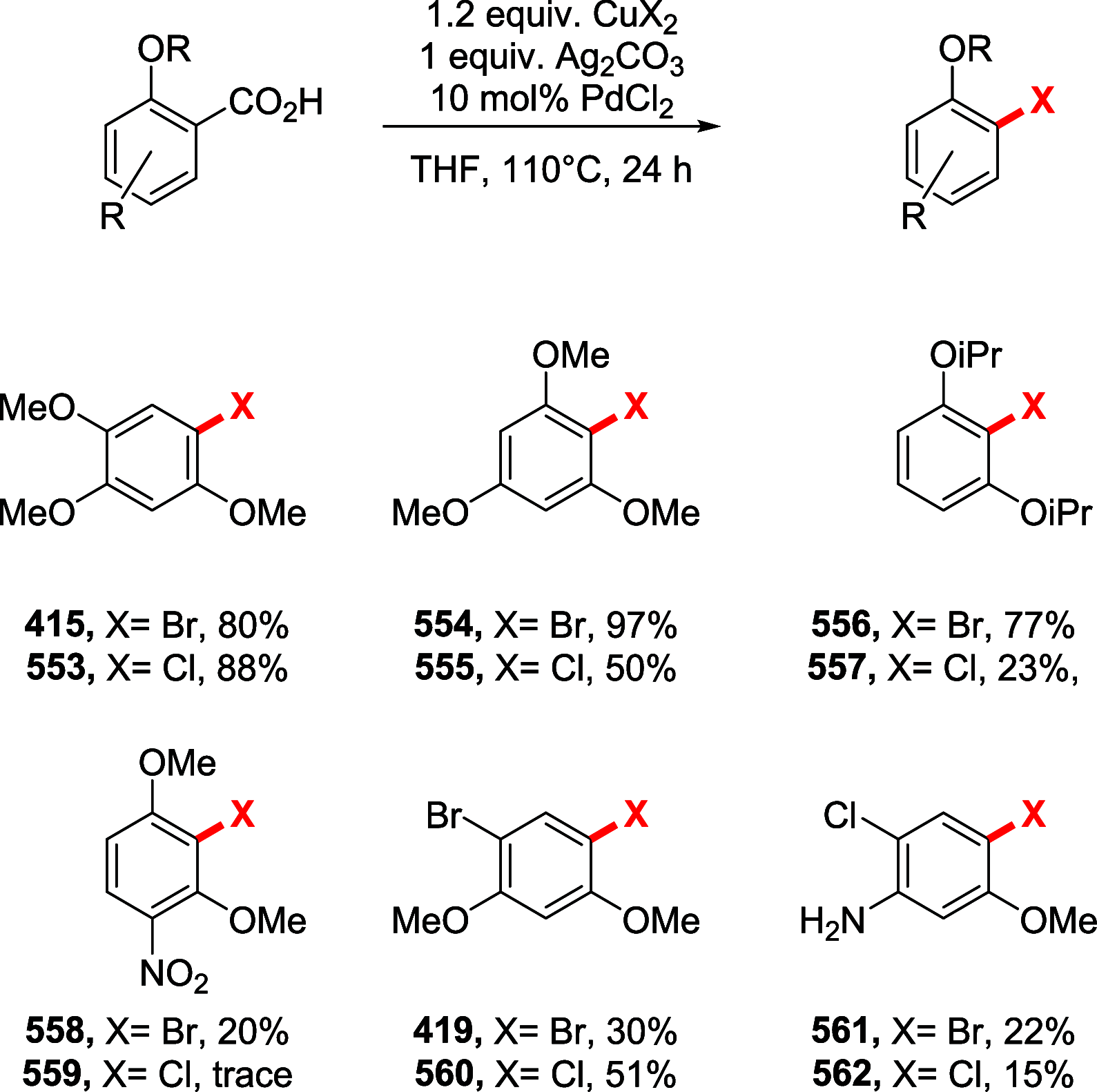
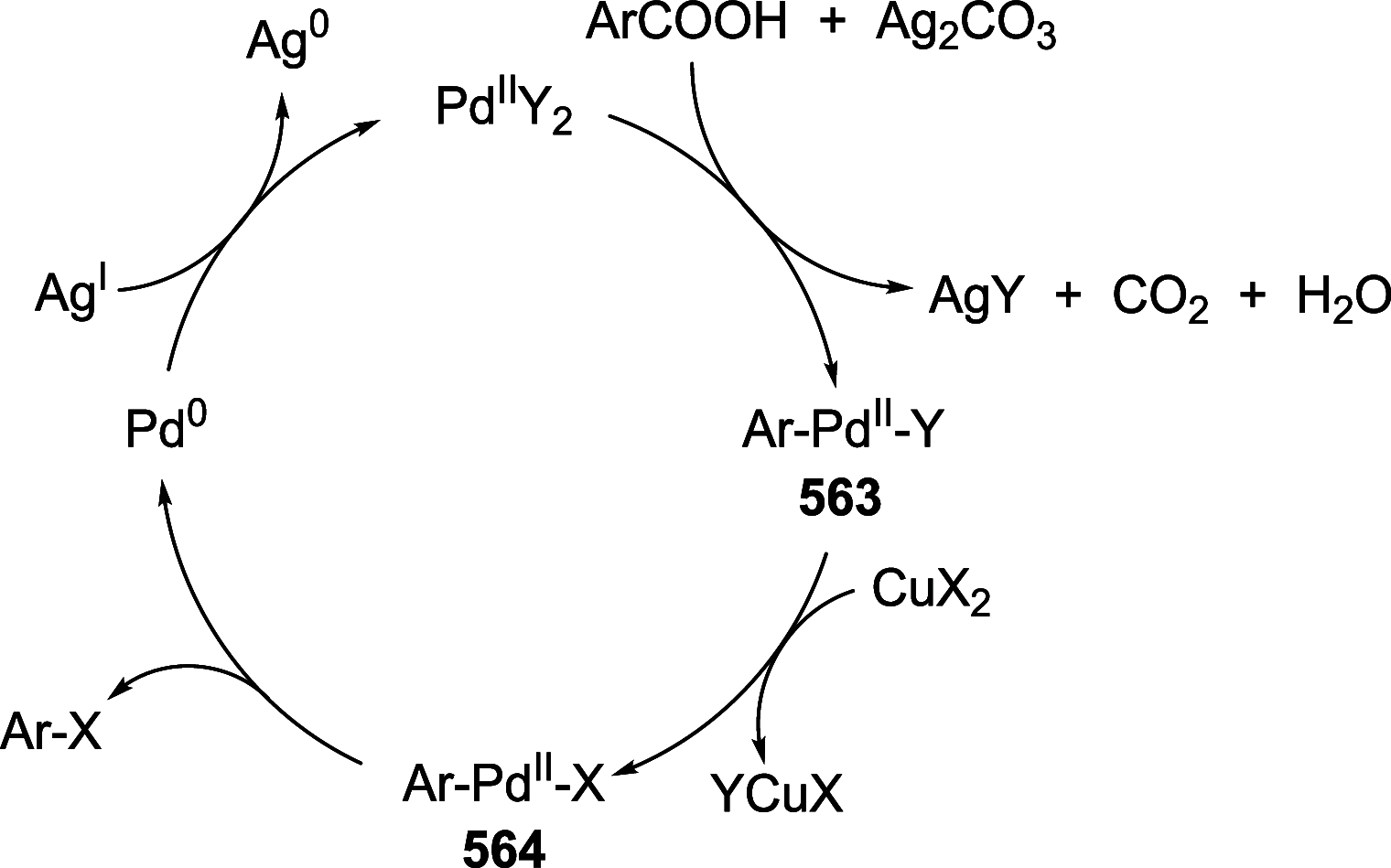


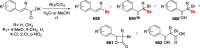

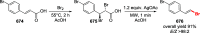

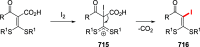


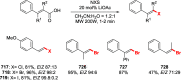

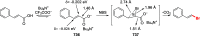
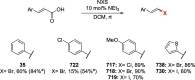

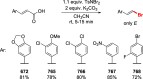
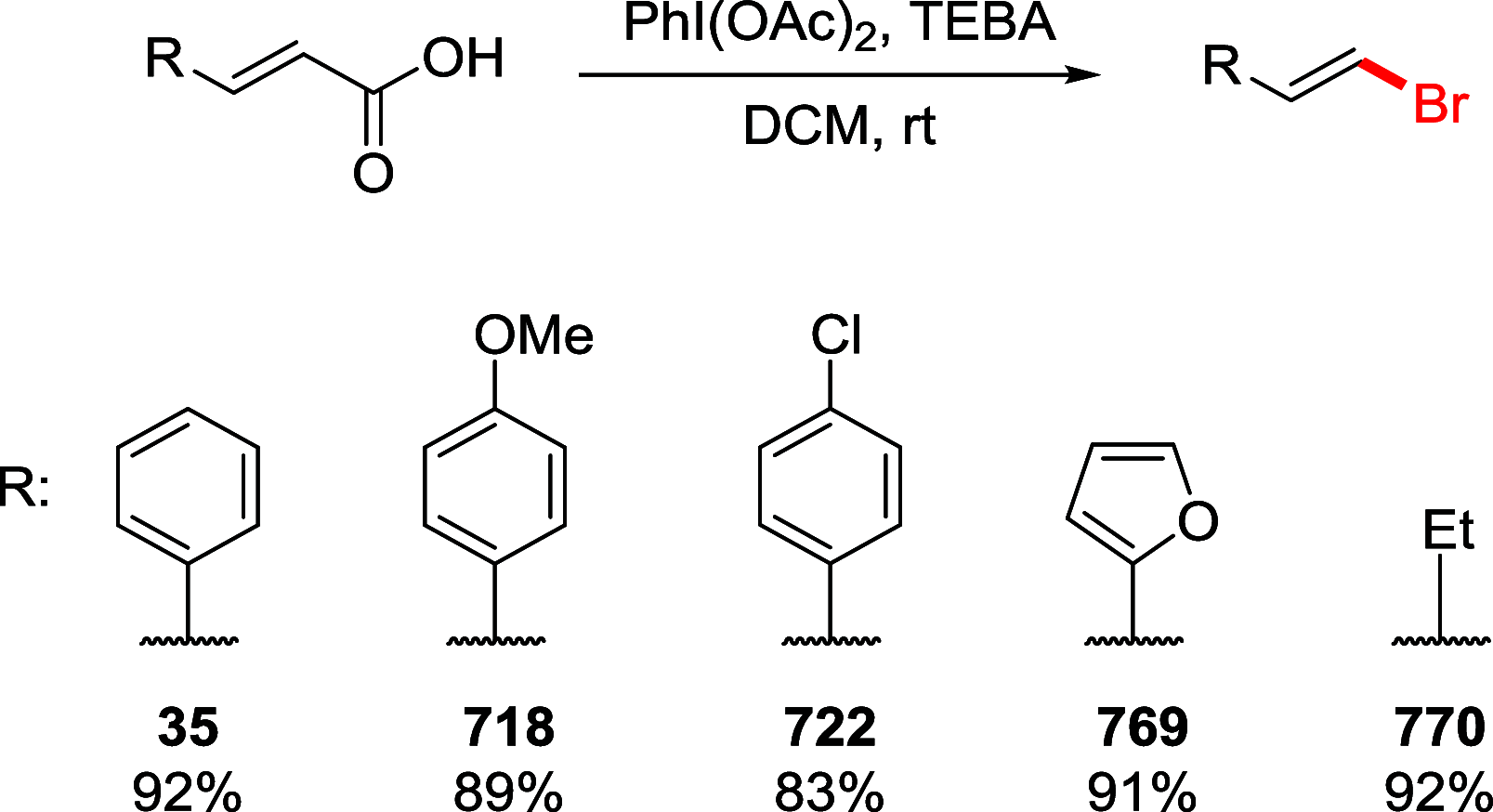
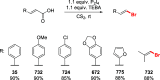
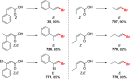


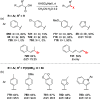
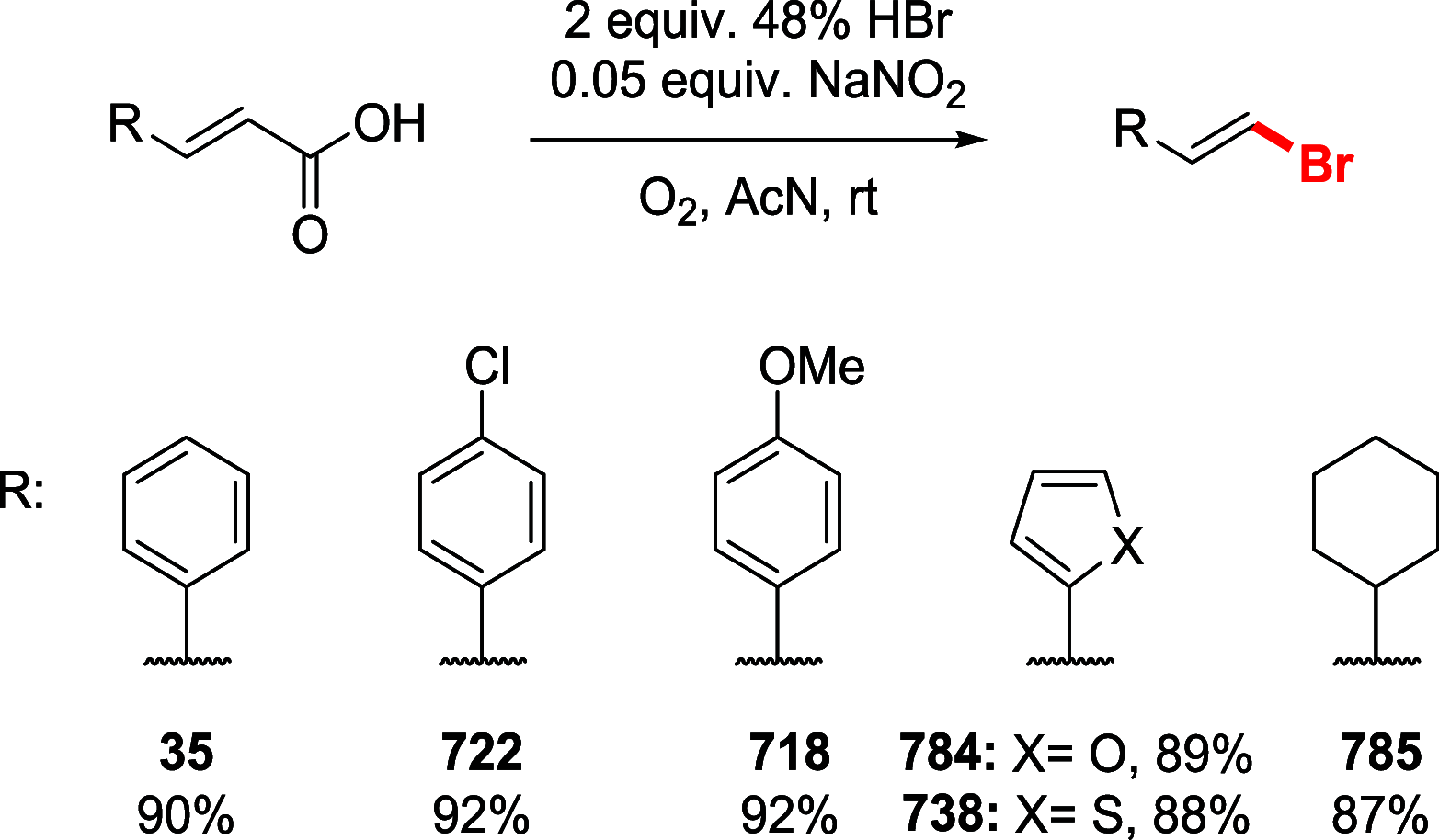
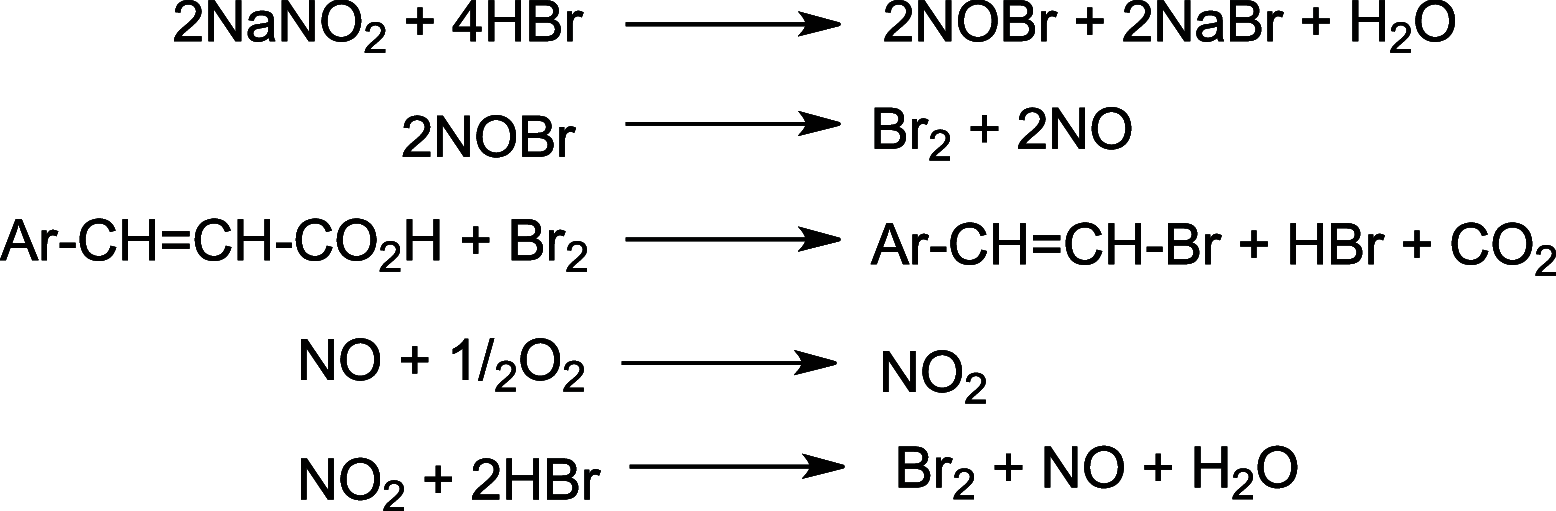

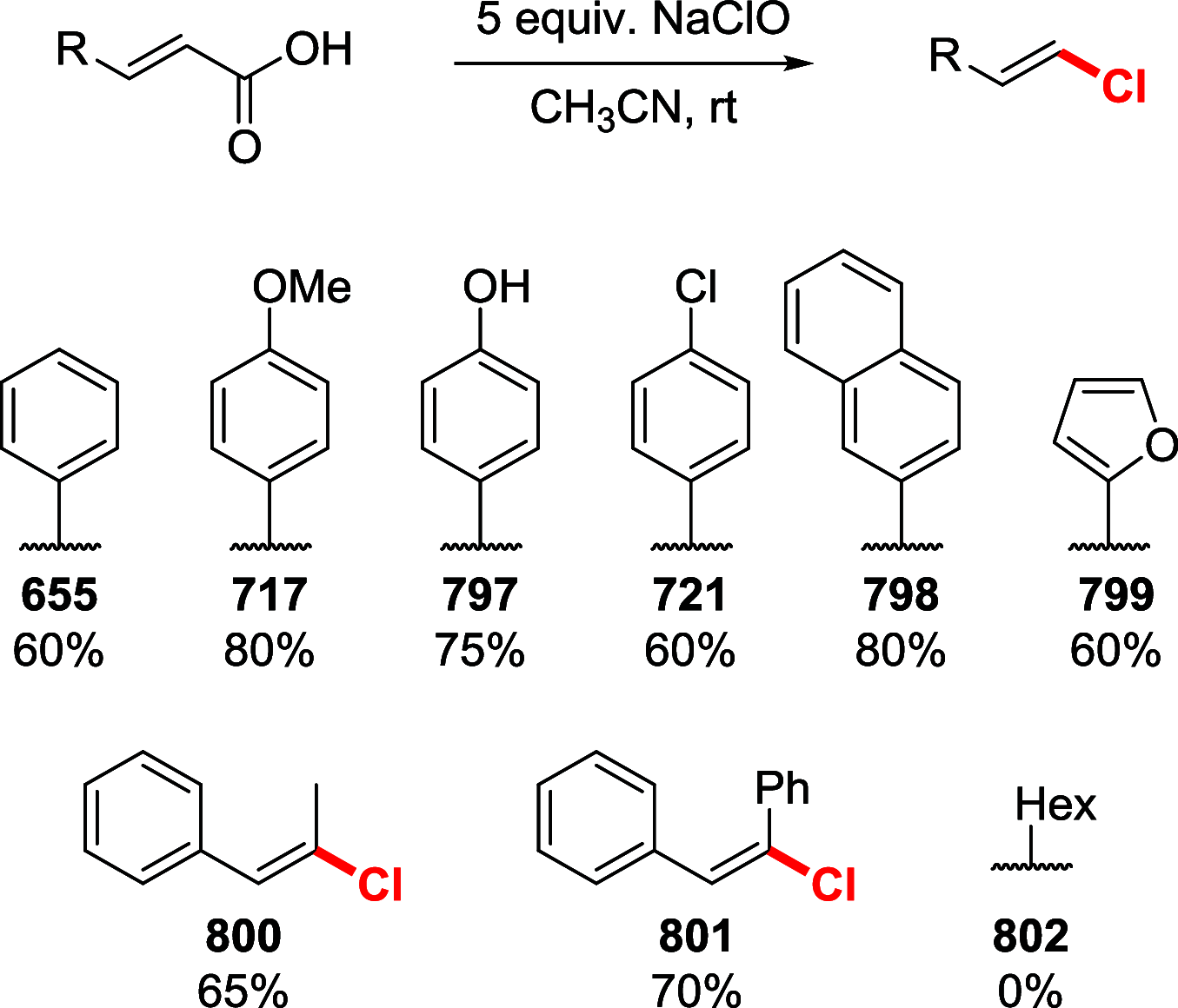
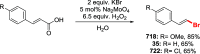

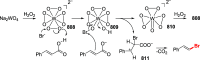
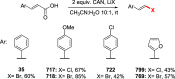
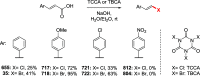
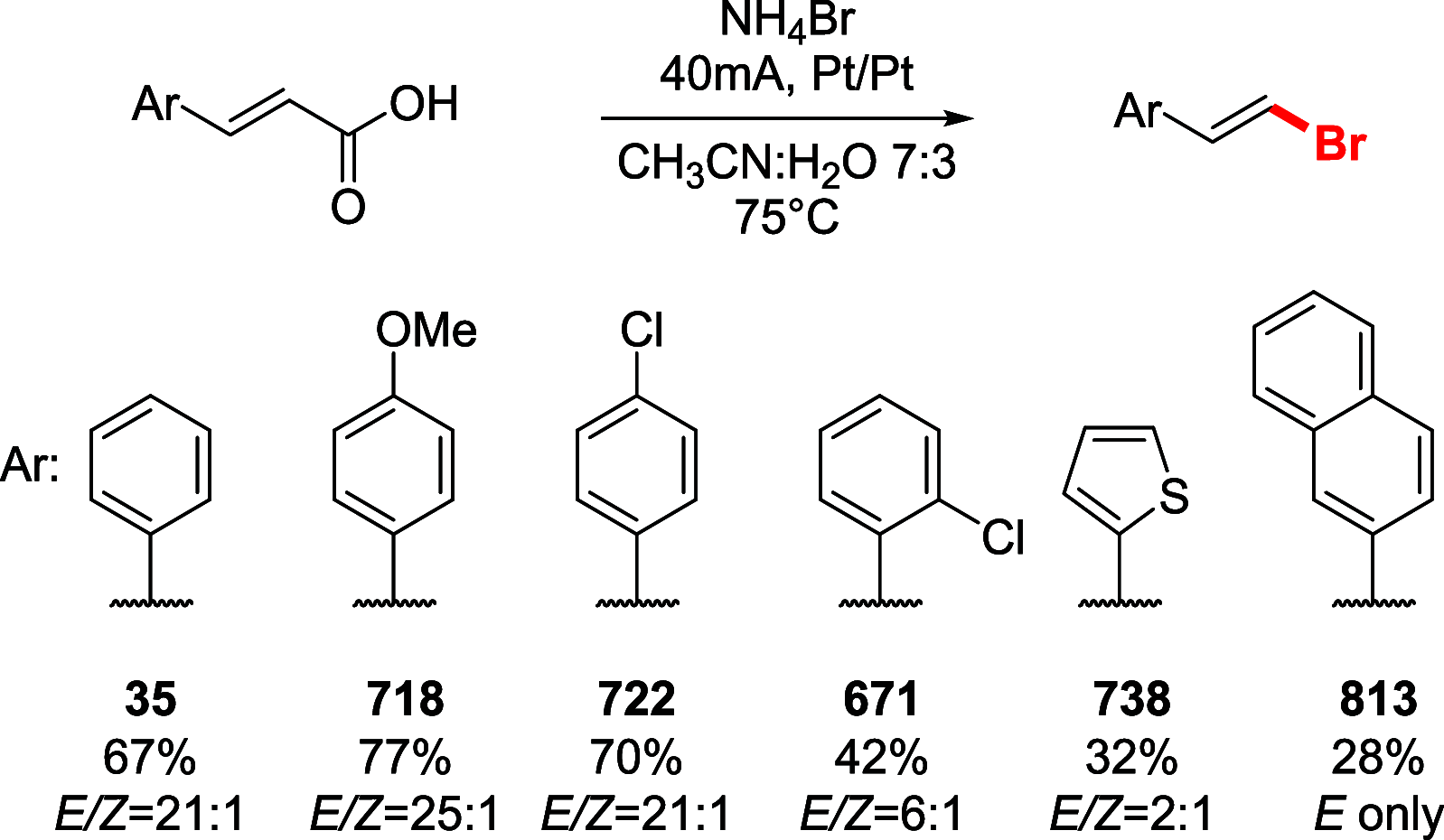
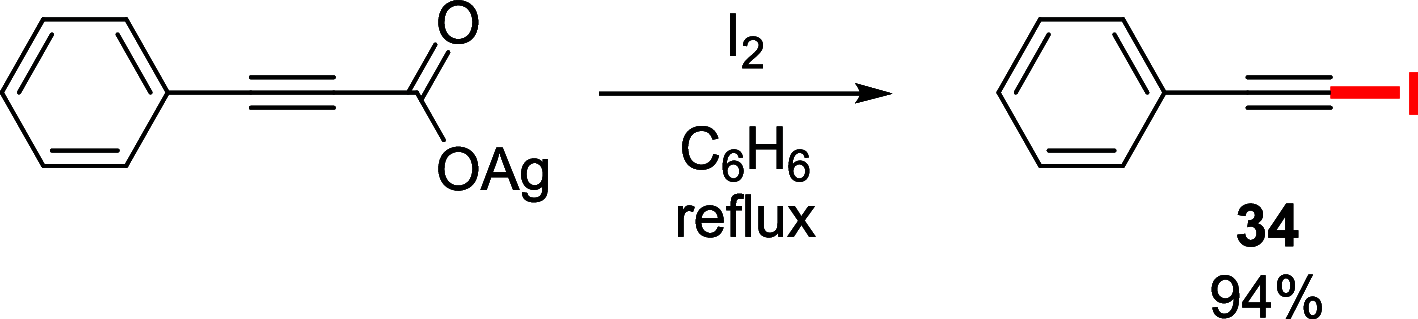

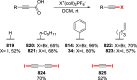


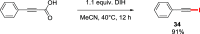

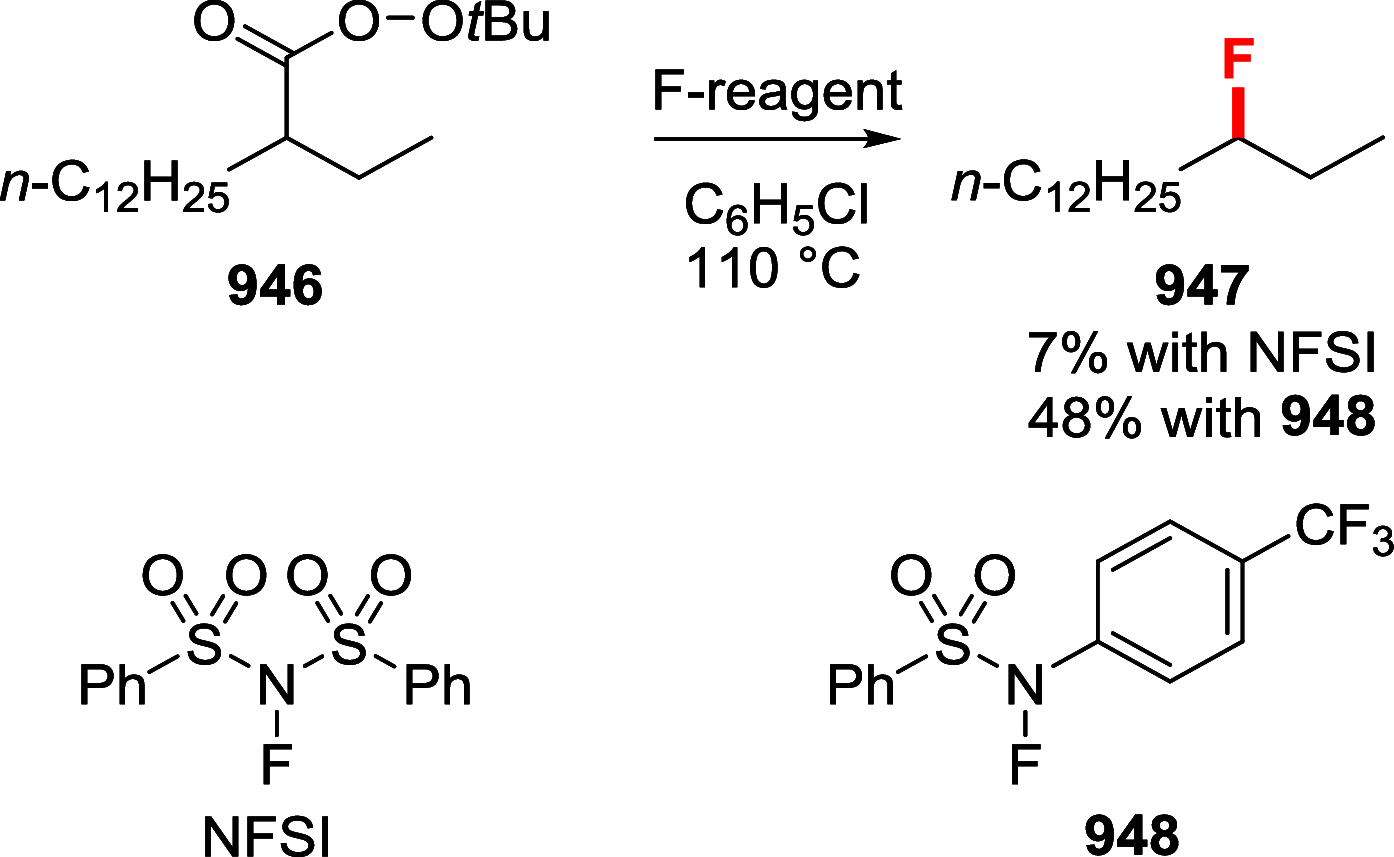
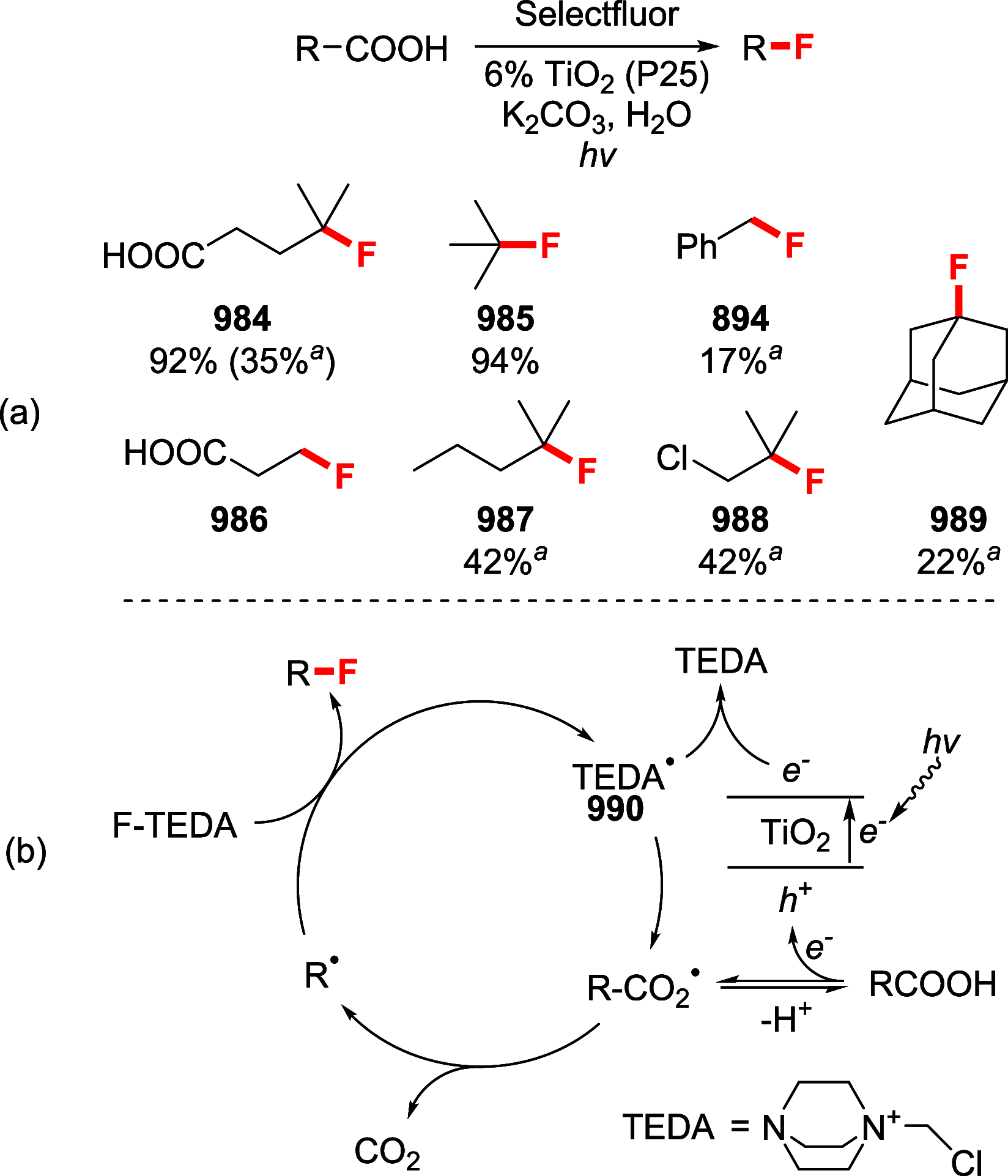
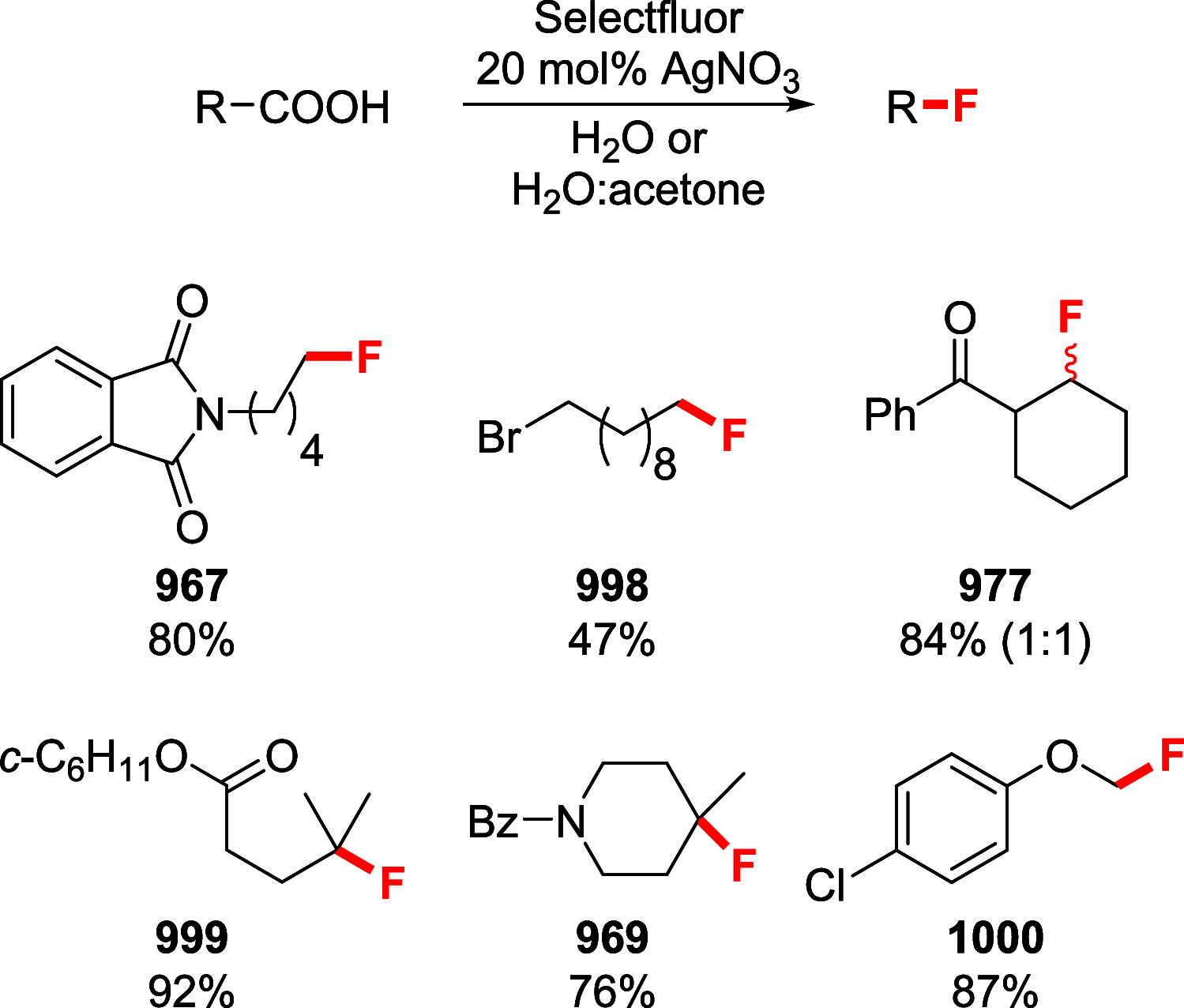
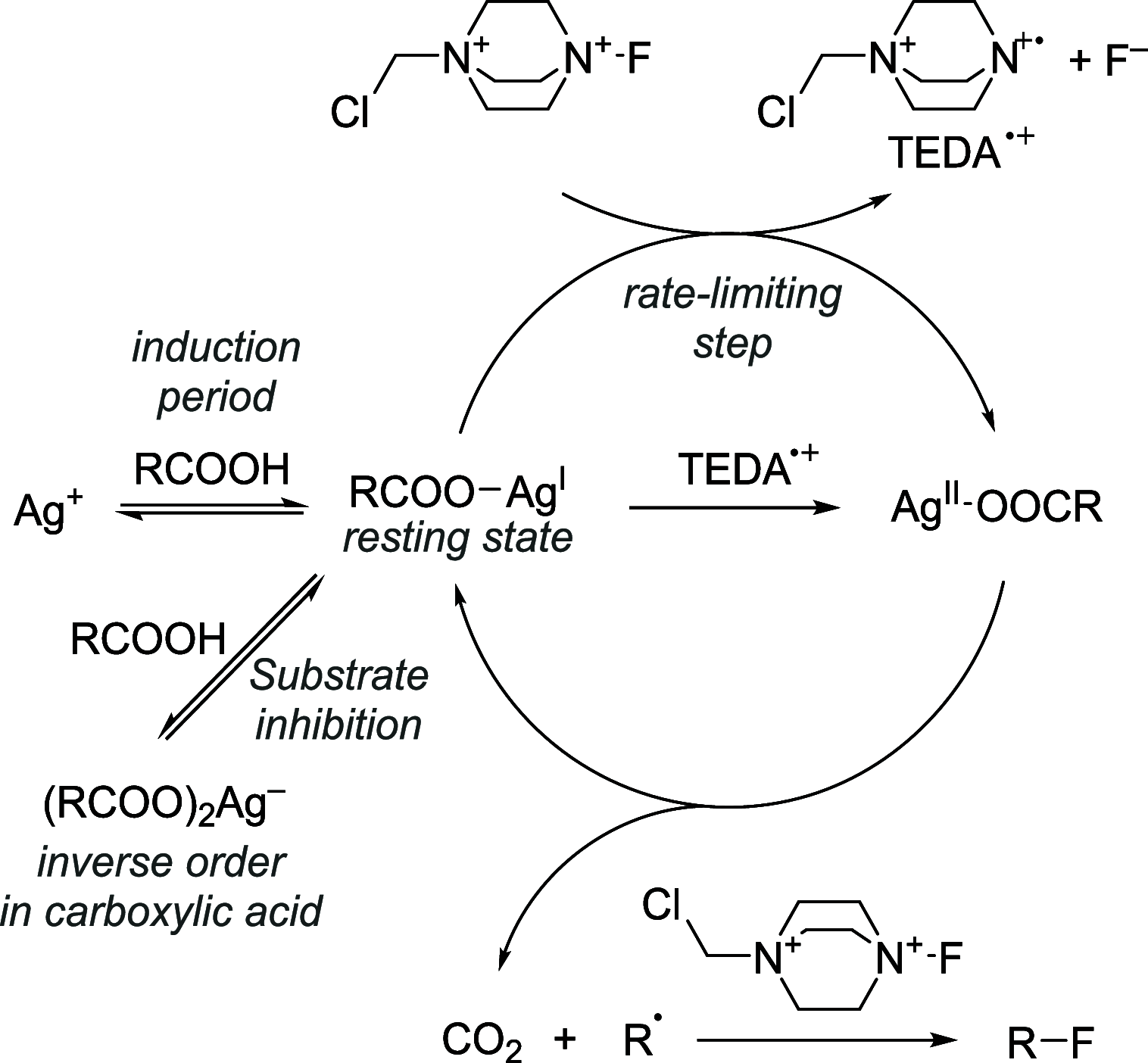





References
-
- Global Demand for PVC to Rise by About 3.2%/Year to 2021. Addit. Polym. 2014, 2014, 10–11.
-
- Zhdankin V. V.; Gribble G. W. Newly Discovered Naturally Occurring Organohalogens. ARKIVOC 2019, 2018, 372–410. 10.24820/ark.5550190.p010.610. - DOI
-
- Sheldon R. A.; Kochi J. K. Oxidative Decarboxylation of Acids by Lead Tetraacetate. In Org. React. 1972, 19, 279–422.
-
- Johnson R. G.; Ingham R. K. The Degradation of Carboxylic Acid Salts by Means of Halogen - the Hunsdiecker Reaction. Chem. Rev. 1956, 56, 219–269. 10.1021/cr50008a002. - DOI
Publication types
LinkOut - more resources
Full Text Sources

