Prophylactic Dressings for Maintaining Skin Integrity of Healthcare Workers When Using N95 Respirators While Preventing Contamination Due to the Novel Coronavirus: A Quality Improvement Project
- PMID: 33201140
- PMCID: PMC7678667
- DOI: 10.1097/WON.0000000000000713
Prophylactic Dressings for Maintaining Skin Integrity of Healthcare Workers When Using N95 Respirators While Preventing Contamination Due to the Novel Coronavirus: A Quality Improvement Project
Abstract
Purpose: Extended use of N95 respirator masks is far more prevalent during the coronavirus disease 2019 (COVID-19) pandemic. As WOC nurses, we were tasked with formulating procedures for protecting the facial skin integrity of healthcare workers (HCWs) using personal protective devices when caring for patients with suspected or active COVID-19, while avoiding contamination when the masks are donned or doffed. This quality improvement project describes how we approached this project within the limited time frame available as we cared for patients with established and suspected COVID-19.
Participants and setting: This project focused on HCW use of N95 respirator masks and dressings currently available in our facility. The 4 WOC nurses acted as quality improvement project directors and as participants. The setting for our project was our facility's simulation laboratory.
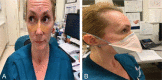
Approach: We evaluated 6 topical products (an alcohol-free liquid acrylate, thin film dressing, thin hydrocolloid dressing, hydrocolloid blister care cushion, thin foam transfer dressing, and thick foam dressing) applied to skin in contact with 3 N95 respirators; all are available on our facility's formulary and all are in widespread clinical use. After the product was applied to the face and nose, the N95 respirator was donned and evaluated for fit. Participants then wore the devices for 10 hours and doffed the mask using established facility procedures. In order to evaluate for potential contamination including possible aerosolization, we applied a commercially available fluorescent lotion to simulate the presence of infectious particles. Contamination was assessed using an ultraviolet light for all dressings except for the alcohol-free liquid acrylate. We also evaluated cutaneous responses (skin integrity, irritation, comfort) during this period.
Outcomes: We found that contamination of the simulated pathogen did not occur with removal of any of the protective products. No skin irritation was noted with any of the tested products after a 10-hour wear time underneath the N95 respirator masks, but mild discomfort was experienced with 3 of the dressings (thin film dressing and both hydrocolloid dressings).
Conclusion: Based on these experiences, we recommend application of an alcohol-free liquid acrylate film to prevent facial skin injury associated with friction from the extended use of an N95 respirator mask. We further recommend performing a fit test and user-performed seal check with the use of any topical dressing and especially those that add cushion. For the duration of the COVID-19 pandemic, we recommend use of protective dressings to maintain skin integrity and protection from coronavirus infection as HCWs continue to provide care to all of patients under their care.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




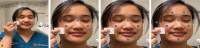





Similar articles
-
Use of Thin Dressings Under N95 Respirators: Exploring Their Effect on Quantitative Fit Testing Results to Guide Hospital Practice During the COVID-19 Pandemic.Wound Manag Prev. 2020 Nov;66(11):13-17. Wound Manag Prev. 2020. PMID: 33206625
-
The need of health policy perspective to protect Healthcare Workers during COVID-19 pandemic. A GRADE rapid review on the N95 respirators effectiveness.PLoS One. 2020 Jun 3;15(6):e0234025. doi: 10.1371/journal.pone.0234025. eCollection 2020. PLoS One. 2020. PMID: 32492045 Free PMC article.
-
Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff.Cochrane Database Syst Rev. 2020 May 15;5(5):CD011621. doi: 10.1002/14651858.CD011621.pub5. Cochrane Database Syst Rev. 2020. PMID: 32412096 Free PMC article.
-
Powered Air Purifying Respirator (PAPR) restores the N95 face mask induced cerebral hemodynamic alterations among Healthcare Workers during COVID-19 Outbreak.J Neurol Sci. 2020 Oct 15;417:117078. doi: 10.1016/j.jns.2020.117078. Epub 2020 Aug 3. J Neurol Sci. 2020. PMID: 32768718 Free PMC article.
-
Facial Skin Temperature and Discomfort When Wearing Protective Face Masks: Thermal Infrared Imaging Evaluation and Hands Moving the Mask.Int J Environ Res Public Health. 2020 Jun 27;17(13):4624. doi: 10.3390/ijerph17134624. Int J Environ Res Public Health. 2020. PMID: 32605056 Free PMC article.
Cited by
-
Skin care and hygiene among healthcare professionals during and after the SARS-CoV-2 pandemic.SAGE Open Med. 2021 Dec 8;9:20503121211062795. doi: 10.1177/20503121211062795. eCollection 2021. SAGE Open Med. 2021. PMID: 34917384 Free PMC article. Review.
-
Impact in Contact Dermatitis during and after SARS-CoV2 Pandemic.Curr Treat Options Allergy. 2022;9(1):19-26. doi: 10.1007/s40521-022-00298-2. Epub 2022 Feb 10. Curr Treat Options Allergy. 2022. PMID: 35194543 Free PMC article. Review.
-
Changes in the use of cosmetics worldwide due to increased use of masks in the coronavirus disease-19 pandemic.J Cosmet Dermatol. 2022 Jul;21(7):2708-2712. doi: 10.1111/jocd.14515. Epub 2022 Apr 24. J Cosmet Dermatol. 2022. PMID: 35466523 Free PMC article. Review.
-
Hydrocolloid dressing versus conventional wound care after dermatologic surgery.JAAD Int. 2021 Dec 21;6:37-42. doi: 10.1016/j.jdin.2021.11.002. eCollection 2022 Mar. JAAD Int. 2021. PMID: 34993497 Free PMC article.
-
Design considerations for protective mask development: A remote mask usability evaluation.Appl Ergon. 2022 Jul;102:103751. doi: 10.1016/j.apergo.2022.103751. Epub 2022 Mar 24. Appl Ergon. 2022. PMID: 35339761 Free PMC article. Clinical Trial.
References
-
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) interim guidance for conserving and extending filtering facepiece respirator supply in non-healthcare sectors. https://www.cdc.gov/coronavirus/2019-ncov/community/conserving-respirato.... Accessed April 23, 2020.
-
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) strategies for optimizing the supply of N95 respirators. https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/index.... Accessed April 23, 2020.
-
- Gefen A, Ousey K. Update to device-related pressure ulcers: SECURE prevention. COVID-19, face masks and skin damage. J Wound Care. 2020;29(5):245–259. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

