In Vivo Evidence for Impaired Glymphatic Function in the Visual Pathway of Patients With Normal Pressure Hydrocephalus
- PMID: 33201186
- PMCID: PMC7683855
- DOI: 10.1167/iovs.61.13.24
In Vivo Evidence for Impaired Glymphatic Function in the Visual Pathway of Patients With Normal Pressure Hydrocephalus
Abstract
Purpose: Impaired ability to remove toxic metabolites from central nervous system may be an important link between cerebral and ophthalmic degenerative diseases. The aim of the present study was to compare the glymphatic function in the visual pathway in patients with idiopathic normal pressure hydrocephalus (iNPH), a neurodegenerative dementia subtype, with a reference group.
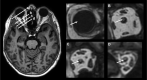
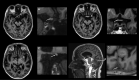
Methods: We compared 31 subjects with Definite iNPH (i.e., shunt-responsive) with 13 references in a prospective and observational study. After intrathecal injection of the magnetic contrast agent gadobutrol (Gadovist, 0.5 mL, 1.0 mmol/mL, Bayer Pharma AG), serving as a tracer, consecutive magnetic resonance imaging (MRI) scans were obtained (next 24-48 hours). The normalized MRI T1 signal recorded in the cerebrospinal fluid (CSF) and along the visual pathway served as a semi-quantitative measure of tracer enrichment. Gadobutrol does not penetrate the blood-brain barrier and is thus confined to the extravascular space. Overnight measurements of pulsatile intracranial pressure were used as a surrogate marker for the intracranial compliance.
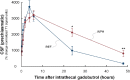
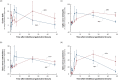
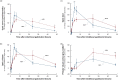
Results: The tracer enriched the prechiasmatic cistern similarly in both groups, but clearance was delayed in the iNPH group. Moreover, both delayed enrichment and clearance of the tracer were observed in the visual pathway in the iNPH subjects. The enrichment in the visual pathway and the CSF correlated. Individuals with elevated pulsatile intracranial pressure showed reduced enrichment within the visual pathway.
Conclusions: There was delayed enrichment and clearance of a tracer in the visual pathway of iNPH patients, which suggests impaired glymphatic function in the visual pathway in this disease.
Conflict of interest statement
Disclosure:
Figures
References
-
- Denniston AK, Keane PA.. Paravascular pathways in the eye: is there an ‘ocular glymphatic system’? Invest Ophthalmol Vis Sci. 2015; 56(6): 3955–3956. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

