Phase I study of liposomal irinotecan in patients with metastatic breast cancer: findings from the expansion phase
- PMID: 33201358
- PMCID: PMC7921078
- DOI: 10.1007/s10549-020-05995-7
Phase I study of liposomal irinotecan in patients with metastatic breast cancer: findings from the expansion phase
Abstract
Purpose: Metastatic breast cancer (mBC) remains incurable and is associated with low survival rates. This study assessed the efficacy and safety of liposomal irinotecan in heavily pretreated patients with mBC, with or without active brain metastases (BM).
Methods: Following the dose escalation phase and determination of recommended phase 2 dose, the expansion phase of this phase I, open-label, non-randomized study, assigned adult women to cohorts based on mBC subtype: cohort 1, hormone receptor +/human epidermal growth factor receptor 2-; cohort 2, triple-negative breast cancer; or cohort 3, any mBC subtype with active BM. Patients received liposomal irinotecan 50 or 70 mg/m2 free base every 2 weeks. Here, we report secondary outcomes including best overall response (BOR), objective response rate (ORR), and treatment-emergent adverse events (TEAEs).
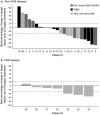
Results: For non-central nervous system (non-CNS) disease across all cohorts (intent-to-treat population, N = 29), the ORR was 34.5% (95% confidence interval: 17.94-54.33), with a BOR of partial response in 10 patients (34.5%), stable disease in five (17.2%), progressive disease in 10 (34.5%); four patients were unevaluable (13.8%). The ORR for the CNS cohort was 30.0% (95% confidence interval: 6.67-65.25) using modified Response Evaluation Criteria in Solid Tumors. Common grade 3 or higher TEAEs were diarrhea (27.6%), nausea (17.2%), fatigue (13.8%), asthenia (10.3%), and hypokalemia (10.3%). Serious treatment-related TEAEs were reported in six patients (20.7%). No treatment-related TEAEs resulted in death.
Conclusions: Liposomal irinotecan monotherapy demonstrated antitumor activity in heavily pretreated patients with mBC, with or without BM. The observed safety profile was consistent with that in previous studies.
Clinical trial registration: Trial registration ID NCT01770353.
Keywords: Brain metastases; Heavily pretreated patients; Liposomal irinotecan; Metastatic breast cancer; Objective response rate; Phase I clinical trial.
Conflict of interest statement
Jasgit C. Sachdev has received research funding from Celgene, Genentech, and Pfizer; compensation for the role of adviser from Celgene, Ipsen, Novartis, Pfizer, Puma Biotechnology, TapImmune, Tempus, and TTC Oncology; and honorarium from Celgene, Ipsen, Novartis, Pfizer, Puma Biotechnology, and Tempus. Hyo Sook Han has received research funding from AbbVie, Bristol Myers Squibb, the Department of Defense, Horizon Therapeutics, Ipsen, Karyopharm Therapeutics, Novartis, Pfizer, Prescient, Seattle Genetics, TapImmune, and Tesaro; and compensation for a speaker’s bureau from Eli Lilly. Cynthia Ma has received research funding from Pfizer, Puma Biotechnology, and Tempus; compensations for a consulting role for Agendia, AstraZeneca, Eli Lilly, Novartis, OncoSignal, Pfizer, Seattle Genetics, and Tempus. Fiona Maxwell, Tiffany Wang, Bruce Belanger, Bin Zhang, Yan Moore, and Arunthathi Thiagalingam are employees of Ipsen and hold stock or stock options. Carey Anders has received research funding from Eli Lilly, G1-Therapeutics, Merck, Nektar Technology, Puma Biotechnology, Seattle Genetics, and Tesaro; compensation for a consultant role from Eisai, Genentech, Ipsen, Puma Biotechnology, and Seattle Genetics; and royalties from Jones & Bartlett Learning and UpToDate. Pamela Munster and Donald W. Northfelt have nothing to disclose.
Figures


References
-
- Cancer.Net (2020) Breast cancer—metastatic: statistics. https://www.cancer.net/cancer-types/breast-cancer-metastatic/statistics. Accessed 7 Sept 2020
-
- Cardoso F, Bedard PL, Winer EP, Pagani O, Senkus-Konefka E, Fallowfield LJ, Kyriakides S, Costa A, Cufer T, Albain KS. International guidelines for management of metastatic breast cancer: combination vs sequential single-agent chemotherapy. J Natl Cancer Inst. 2009;101(17):1174–1181. doi: 10.1093/jnci/djp235. - DOI - PMC - PubMed
-
- Seattle Genetics Inc. (2020) Prescribing information, TUKYSATM (tucatinib) tablets, for oral use. US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213411s000lbl.pdf. Accessed 9 Sept 2020
-
- Ipsen Biopharmaceuticals, Inc. (2017) Prescribing Information, ONIVYDE® (irinotecan liposome injection). US Food and Drug Administration. https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/sites/9/2.... Accessed 12 Aug 2019
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

