Endothelium-specific deletion of Nox4 delays retinal vascular development and mitigates pathological angiogenesis
- PMID: 33201372
- PMCID: PMC8126573
- DOI: 10.1007/s10456-020-09757-3
Endothelium-specific deletion of Nox4 delays retinal vascular development and mitigates pathological angiogenesis
Abstract
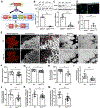
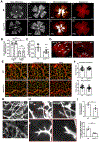
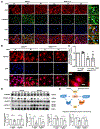
NADPH oxidase 4 (Nox4) is a major isoform of NADPH oxidases playing an important role in many biological processes. Previously we have shown that Nox4 is highly expressed in retinal blood vessels and is upregulated in oxygen-induced retinopathy (OIR). However, the exact role of endothelial Nox4 in retinal angiogenesis remains elusive. Herein, using endothelial cell (EC)-specific Nox4 knockout (Nox4EC-KO) mice, we investigated the impact of endothelial Nox4 deletion on retinal vascular development and pathological angiogenesis during OIR. Our results show that deletion of Nox4 in ECs led to retarded retinal vasculature development with fewer, blunted-end tip cells and sparser, dysmorphic filopodia at vascular front, and reduced density of vascular network in superficial, deep, and intermediate layers in postnatal day 7 (P7), P12, and P17 retinas, respectively. In OIR, loss of endothelial Nox4 had no effect on hyperoxia-induced retinal vaso-obliteration at P9 but significantly reduced aberrant retinal neovascularization at P17 and decreased the deep layer capillary density at P25. Ex vivo study confirmed that lack of Nox4 in ECs impaired vascular sprouting. Mechanistically, loss of Nox4 significantly reduced expression of VEGF, p-VEGFR2, integrin αV, angiopoietin-2, and p-ERK1/2, attenuating EC migration and proliferation. Taken together, our results indicate that endothelial Nox4 is important for retinal vascular development and contributes to pathological angiogenesis, likely through regulation of VEGF/VEGFR2 and angiopoietin-2/integrin αV/ERK pathways. In addition, our study suggests that endothelial Nox4 appears to be essential for intraretinal revascularization after hypoxia. These findings call for caution on targeting endothelial Nox4 in ischemic/hypoxic retinal diseases.
Keywords: Angiogenesis; Development; Endothelial cells; Nox4; Retina; Vascularization.
Conflict of interest statement
Conflict of Interests
The authors declare that they have no conflict of interests.
Figures



Similar articles
-
Endothelial NADPH oxidase 4 mediates vascular endothelial growth factor receptor 2-induced intravitreal neovascularization in a rat model of retinopathy of prematurity.Mol Vis. 2014 Mar 3;20:231-41. eCollection 2014. Mol Vis. 2014. PMID: 24623966 Free PMC article.
-
Functional analyses of TNFR2 in physiological and pathological retina angiogenesis.Invest Ophthalmol Vis Sci. 2013 Jan 9;54(1):211-21. doi: 10.1167/iovs.12-10364. Invest Ophthalmol Vis Sci. 2013. PMID: 23188724 Free PMC article.
-
NADPH oxidase 4-derived H2O2 promotes aberrant retinal neovascularization via activation of VEGF receptor 2 pathway in oxygen-induced retinopathy.J Diabetes Res. 2015;2015:963289. doi: 10.1155/2015/963289. Epub 2015 Mar 18. J Diabetes Res. 2015. PMID: 25866826 Free PMC article.
-
Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review.
-
Redox signaling in angiogenesis: role of NADPH oxidase.Cardiovasc Res. 2006 Jul 15;71(2):226-35. doi: 10.1016/j.cardiores.2006.04.015. Epub 2006 May 9. Cardiovasc Res. 2006. PMID: 16781692 Review.
Cited by
-
The Effects of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase and Erythropoietin, and Their Interactions in Angiogenesis: Implications in Retinopathy of Prematurity.Cells. 2022 Jun 17;11(12):1951. doi: 10.3390/cells11121951. Cells. 2022. PMID: 35741081 Free PMC article. Review.
-
NADPH Oxidases in Pain Processing.Antioxidants (Basel). 2022 Jun 14;11(6):1162. doi: 10.3390/antiox11061162. Antioxidants (Basel). 2022. PMID: 35740059 Free PMC article. Review.
-
Nanoscale coordination polymer Fe-DMY downregulating Poldip2-Nox4-H2O2 pathway and alleviating diabetic retinopathy.J Pharm Anal. 2023 Nov;13(11):1326-1345. doi: 10.1016/j.jpha.2023.05.002. Epub 2023 May 12. J Pharm Anal. 2023. PMID: 38174114 Free PMC article.
-
The Generation of Nitric Oxide from Aldehyde Dehydrogenase-2: The Role of Dietary Nitrates and Their Implication in Cardiovascular Disease Management.Int J Mol Sci. 2022 Dec 7;23(24):15454. doi: 10.3390/ijms232415454. Int J Mol Sci. 2022. PMID: 36555095 Free PMC article. Review.
-
Nutraceuticals/Drugs Promoting Mitophagy and Mitochondrial Biogenesis May Combat the Mitochondrial Dysfunction Driving Progression of Dry Age-Related Macular Degeneration.Nutrients. 2022 May 9;14(9):1985. doi: 10.3390/nu14091985. Nutrients. 2022. PMID: 35565950 Free PMC article. Review.
References
-
- Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H (2010) Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol 12 (10):943–953. doi:10.1038/ncb2103 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

