Outcome and risk factor of immune-related adverse events and pneumonitis in patients with advanced or postoperative recurrent non-small cell lung cancer treated with immune checkpoint inhibitors
- PMID: 33201587
- PMCID: PMC7812074
- DOI: 10.1111/1759-7714.13736
Outcome and risk factor of immune-related adverse events and pneumonitis in patients with advanced or postoperative recurrent non-small cell lung cancer treated with immune checkpoint inhibitors
Abstract
Background: Non-small cell lung cancer (NSCLC) patients with pre-existing respiratory diseases have been excluded in clinical trials of immune checkpoint inhibitor (ICI) therapy, and it is unknown whether the same degree of response can be expected as that in patients without pre-existing respiratory diseases and if they are associated with increased risk for various immune-related adverse events (irAEs) and ICI pneumonitis. This study aimed to evaluate predictive factors of clinical response, prognostic factors, risk factors of irAEs, and ICI pneumonitis in NSCLC patients with or without pre-existing respiratory diseases.
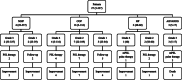
Methods: We conducted a retrospective study of 180 NSCLC patients who received ICI monotherapy of nivolumab, pembrolizumab, or atezolizumab from 1 January 2016 to 31 March 2019.
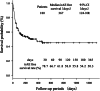
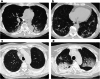
Results: A total of 119 patients had pre-existing respiratory diseases, including 20 with pre-existing idiopathic interstitial pneumonias (IIPs). A total of 85 patients experienced irAEs, of which ICI pneumonitis was the most frequent adverse event, occurring in 27 patients. Of the three patients who died from irAEs, all from ICI pneumonitis, two had pulmonary emphysema and one had pre-existing IIP. In multivariate analyses, irAEs were associated with objective response rate (ORR) and favorable OS, and IIPs were associated with increased risk for ICI pneumonitis. However, IIPs were not associated with low ORR or poor OS.
Conclusions: Pre-existing IIPs were a risk factor for ICI pneumonitis. However, this study showed that ICI therapy can be offered to patients with pre-existing respiratory diseases with the expectation of the same degree of response as that in patients without pre-existing respiratory diseases.
Key points: Significant findings of the study: Pre-existing IIPs were a risk factor for ICI pneumonitis, but objective response rate and prognosis of patients with IIPs were similar to those of other patients.
What this study adds: In patients with pre-existing IIPs, ICI pneumonitis should be noted. However, ICI therapy can be offered to patients with pre-existing respiratory diseases with the expectation of the same degree of response as that in patients without pre-existing respiratory diseases.
Keywords: Immune checkpoint inhibitor; immune-related adverse event; lung cancer; pneumonitis.
© 2020 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures

Similar articles
-
Pre-existing Interstitial Lung Abnormalities and Immune Checkpoint Inhibitor-Related Pneumonitis in Solid Tumors: A Retrospective Analysis.Oncologist. 2024 Jan 5;29(1):e108-e117. doi: 10.1093/oncolo/oyad187. Oncologist. 2024. PMID: 37590388 Free PMC article.
-
Does Pre-Existing Chronic Obstructive Pulmonary Disease Increase the Risk of Checkpoint Inhibitor Pneumonitis in Advanced/Metastatic Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors?Curr Oncol. 2025 Apr 29;32(5):259. doi: 10.3390/curroncol32050259. Curr Oncol. 2025. PMID: 40422518 Free PMC article.
-
High incidence of immune checkpoint inhibitor-induced pneumonitis in patients with non-small cell lung cancer and interstitial pneumonia, regardless of honeycomb lung or forced vital capacity: results from a multicenter retrospective study.Int J Clin Oncol. 2025 May;30(5):904-913. doi: 10.1007/s10147-025-02732-2. Epub 2025 Mar 8. Int J Clin Oncol. 2025. PMID: 40056277
-
Treatment- and immune-related adverse events of immune checkpoint inhibitors in advanced lung cancer.Biosci Rep. 2020 May 29;40(5):BSR20192347. doi: 10.1042/BSR20192347. Biosci Rep. 2020. PMID: 32315071 Free PMC article.
-
Efficacy and safety of immune checkpoint inhibitor consolidation after chemoradiation in patients of Asian ethnicity with unresectable stage III non-small cell lung cancer: Chinese multicenter report and literature review.Thorac Cancer. 2020 Oct;11(10):2916-2923. doi: 10.1111/1759-7714.13631. Epub 2020 Aug 24. Thorac Cancer. 2020. PMID: 32833338 Free PMC article. Review.
Cited by
-
Non-bacterial cystitis caused by pembrolizumab therapy for adenocarcinoma of the lung: a case report.Front Immunol. 2024 Jul 5;15:1423123. doi: 10.3389/fimmu.2024.1423123. eCollection 2024. Front Immunol. 2024. PMID: 39034999 Free PMC article.
-
Chinese expert consensus on the multidisciplinary management of pneumonitis associated with immune checkpoint inhibitor.Thorac Cancer. 2022 Dec;13(23):3420-3430. doi: 10.1111/1759-7714.14693. Epub 2022 Oct 21. Thorac Cancer. 2022. PMID: 36268845 Free PMC article.
-
Biomarkers and risk factors for the early prediction of immune-related adverse events: a review.Hum Vaccin Immunother. 2022 Dec 31;18(1):2018894. doi: 10.1080/21645515.2021.2018894. Epub 2022 Feb 2. Hum Vaccin Immunother. 2022. PMID: 35108160 Free PMC article. Review.
-
Early presentation of pembrolizumab-associated pneumonitis.BMJ Case Rep. 2021 Jul 15;14(7):e242493. doi: 10.1136/bcr-2021-242493. BMJ Case Rep. 2021. PMID: 34266820 Free PMC article.
-
Treatment Strategies for Non-Small-Cell Lung Cancer with Comorbid Respiratory Disease; Interstitial Pneumonia, Chronic Obstructive Pulmonary Disease, and Tuberculosis.Cancers (Basel). 2024 Apr 29;16(9):1734. doi: 10.3390/cancers16091734. Cancers (Basel). 2024. PMID: 38730686 Free PMC article. Review.
References
-
- Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387: 1540–50. - PubMed
-
- Reck M, Rodriguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

