An Electronically Delivered Patient-Activation Tool for Intensification of Medications for Chronic Heart Failure With Reduced Ejection Fraction: The EPIC-HF Trial
- PMID: 33201741
- PMCID: PMC7855616
- DOI: 10.1161/CIRCULATIONAHA.120.051863
An Electronically Delivered Patient-Activation Tool for Intensification of Medications for Chronic Heart Failure With Reduced Ejection Fraction: The EPIC-HF Trial
Abstract
Background: Major gaps exist in the routine initiation and dose up-titration of guideline-directed medical therapies (GDMT) for patients with heart failure with reduced ejection fraction. Without novel approaches to improve prescribing, the cumulative benefits of heart failure with reduced ejection fraction treatment will be largely unrealized. Direct-to-consumer marketing and shared decision making reflect a culture where patients are increasingly involved in treatment choices, creating opportunities for prescribing interventions that engage patients.
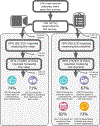
Methods: The EPIC-HF (Electronically Delivered, Patient-Activation Tool for Intensification of Medications for Chronic Heart Failure with Reduced Ejection Fraction) trial randomized patients with heart failure with reduced ejection fraction from a diverse health system to usual care versus patient activation tools-a 3-minute video and 1-page checklist-delivered electronically 1 week before, 3 days before, and 24 hours before a cardiology clinic visit. The tools encouraged patients to work collaboratively with their clinicians to "make one positive change" in heart failure with reduced ejection fraction prescribing. The primary endpoint was the percentage of patients with GDMT medication initiations and dose intensifications from immediately preceding the cardiology clinic visit to 30 days after, compared with usual care during the same period.
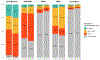
Results: EPIC-HF enrolled 306 patients, 290 of whom attended a clinic visit during the study period: 145 were sent the patient activation tools and 145 were controls. The median age of patients was 65 years; 29% were female, 11% were Black, 7% were Hispanic, and the median ejection fraction was 32%. Preclinic data revealed significant GDMT opportunities, with no patients on target doses of β-blocker, sacubitril/valsartan, and mineralocorticoid receptor antagonists. From immediately preceding the cardiology clinic visit to 30 days after, 49.0% in the intervention and 29.7% in the control experienced an initiation or intensification of their GDMT (P=0.001). The majority of these changes were made at the clinician encounter itself and involved dose uptitrations. There were no deaths and no significant differences in hospitalization or emergency department visits at 30 days between groups.
Conclusions: A patient activation tool delivered electronically before a cardiology clinic visit improved clinician intensification of GDMT. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03334188.
Keywords: clinical trial; decision making, shared; evidence-based medicines; heart failure; patient participation; prescriptions; quality of health care.
Conflict of interest statement
Conflicts of Interest
Dr. Allen reports grant funding from AHA, NIH, and PCORI, and reports consulting fees from Abbott, ACI Clinical, Amgen, Boston Scientific, Cytokinetics, and Novartis.
Ms. Venechuk reports no disclosures.
Dr. McIlvennan reports no disclosures.
Dr. Page reports no disclosures.
Dr. Knoepke is supported by the NIH (1K23HL153892) and the American Heart Association (18CDA34110026).
Ms. Helmkamp reports no disclosures.
Dr. Khazanie is supported by the NIH and NIH Ethics Supplement (K23HL145122) and the Doris Duke-University of Colorado FRCS Award.
Dr. Peterson reports grant funding from NHLBI (4R33HL143324–02).
Mr. Pierce reports no disclosures.
Mr. Harger reports no disclosures.
Ms. Thompson reports no disclosures.
Dr. Dow reports no disclosures.
Dr. Richards reports no disclosures.
Dr. Huang reports no disclosures.
Dr. Strader reports no disclosures.
Dr. Trinkley is supported by the NIH (K12HL137862).
Dr. Kao reports grant funding from NIH, AHA and CDC and reports an advisory agreement with Codex, Inc.
Dr. Magid reports grant funding form NIH, AHA, and CMS.
Dr. Buttrick reports grant funding from AHA.
Dr. Matlock reports funding from AHA, NIH, and PCORI.
Figures


References
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. - PubMed
-
- Yancy CW, Januzzi JL Jr., Allen LA, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Jessup M, Lindenfeld J, Maddox TM, et al. 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;71:201–230. - PubMed
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. - PubMed
-
- Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72:351–366. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

