Chest CT in the Emergency Department for Diagnosis of COVID-19 Pneumonia: Dutch Experience
- PMID: 33201791
- PMCID: PMC7676748
- DOI: 10.1148/radiol.2020203465
Chest CT in the Emergency Department for Diagnosis of COVID-19 Pneumonia: Dutch Experience
Abstract
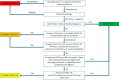
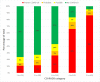
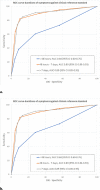
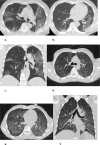
Background Clinicians need to rapidly and reliably diagnose coronavirus disease 2019 (COVID-19) for proper risk stratification, isolation strategies, and treatment decisions. Purpose To assess the real-life performance of radiologist emergency department chest CT interpretation for diagnosing COVID-19 during the acute phase of the pandemic, using the COVID-19 Reporting and Data System (CO-RADS). Materials and Methods This retrospective multicenter study included consecutive patients who presented to emergency departments in six medical centers between March and April 2020 with moderate to severe upper respiratory symptoms suspicious for COVID-19. As part of clinical practice, chest CT scans were obtained for primary work-up and scored using the five-point CO-RADS scheme for suspicion of COVID-19. CT was compared with severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction (RT-PCR) assay and a clinical reference standard established by a multidisciplinary group of clinicians based on RT-PCR, COVID-19 contact history, oxygen therapy, timing of RT-PCR testing, and likely alternative diagnosis. Performance of CT was estimated using area under the receiver operating characteristic curve (AUC) analysis and diagnostic odds ratios against both reference standards. Subgroup analysis was performed on the basis of symptom duration grouped presentations of less than 48 hours, 48 hours through 7 days, and more than 7 days. Results A total of 1070 patients (median age, 66 years; interquartile range, 54-75 years; 626 men) were included, of whom 536 (50%) had a positive RT-PCR result and 137 (13%) of whom were considered to have a possible or probable COVID-19 diagnosis based on the clinical reference standard. Chest CT yielded an AUC of 0.87 (95% CI: 0.84, 0.89) compared with RT-PCR and 0.87 (95% CI: 0.85, 0.89) compared with the clinical reference standard. A CO-RADS score of 4 or greater yielded an odds ratio of 25.9 (95% CI: 18.7, 35.9) for a COVID-19 diagnosis with RT-PCR and an odds ratio of 30.6 (95% CI: 21.1, 44.4) with the clinical reference standard. For symptom duration of less than 48 hours, the AUC fell to 0.71 (95% CI: 0.62, 0.80; P < .001). Conclusion Chest CT analysis using the coronavirus disease 2019 (COVID-19) Reporting and Data System enables rapid and reliable diagnosis of COVID-19, particularly when symptom duration is greater than 48 hours. © RSNA, 2020 Online supplemental material is available for this article. See also the editorial by Elicker in this issue.
Keywords: AUC = area under the receiver operating characteristic curve; CO-RADS = COVID-19 Reporting and Data System; COVID-19 = coronavirus disease 2019; RT-PCR = reverse-transcription polymerase chain reaction; SARS-CoV-2 = severe acute respiratory coronavirus 2.
Figures


Comment in
-
What Is the Performance and Role of CT in Suspected COVID-19 Infection?Radiology. 2021 Feb;298(2):E109-E111. doi: 10.1148/radiol.20202040130. Epub 2020 Nov 17. Radiology. 2021. PMID: 33206006 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

