Clinical Impact of Pretreatment Human Immunodeficiency Virus Drug Resistance in People Initiating Nonnucleoside Reverse Transcriptase Inhibitor-Containing Antiretroviral Therapy: A Systematic Review and Meta-analysis
- PMID: 33202025
- PMCID: PMC8328216
- DOI: 10.1093/infdis/jiaa683
Clinical Impact of Pretreatment Human Immunodeficiency Virus Drug Resistance in People Initiating Nonnucleoside Reverse Transcriptase Inhibitor-Containing Antiretroviral Therapy: A Systematic Review and Meta-analysis
Abstract
Background: Increased access to antiretroviral therapy (ART) has resulted in rising levels of pretreatment human immunodeficiency virus drug resistance (PDR). This is the first systematic review and meta-analysis to assess the impact of PDR on treatment outcomes among people initiating nonnucleoside reverse transcriptase inhibitor (NNRTI)-based ART, including the combination of efavirenz (EFV), tenofovir (TDF), and lamivudine or emtricitabine (XTC).
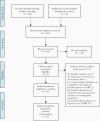
Methods: We systematically reviewed studies and conference proceedings comparing treatment outcomes in populations initiating NNRTI-based ART with and without PDR. We conducted subgroup analyses by regimen: (1) NNRTIs + 2 nucleoside reverse transcriptase inhibitors (NRTIs), (2) EFV + 2 NRTIs, or (3) EFV/TDF/XTC; by population (children vs adults); and by definition of resistance (PDR vs NNRTI PDR).
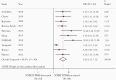
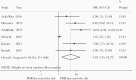
Results: Among 6197 studies screened, 32 were analyzed (31 441 patients). We found that individuals with PDR initiating NNRTIs across all the subgroups had increased risk of virological failure compared to those without PDR. Risk of acquisition of new resistance mutations and ART switch was also higher in people with PDR.
Conclusions: This review shows poorer treatment outcomes in the presence of PDR, supporting the World Health Organization's recommendation to avoid using NNRTIs in countries where levels of PDR are high.
Keywords: ART; HIV drug resistance; NNRTIs; pretreatment HIV drug resistance; treatment failure; virological failure.
© World Health Organization, 2020. All rights reserved. The World Health Organization has granted the Publisher permission for the reproduction of this article.
Figures



References
-
- World Health Organization. HIV drug resistance report. 2019. Available at: https://www.who.int/hiv/pub/drugresistance/hivdr-report-2019/en/. Accessed 10 January 2020.
-
- World Health Organization. Surveillance of HIV drug resistance in adults initiating antiretroviral therapy.http://www.who.int/hiv/pub/drugresistance/pretreatment_drugresistance/en/. Accessed 10 January 2020.
-
- World Health Organization. Guidelines on the public health response to pretreatment HIV drug resistance. https://bit.ly/2C5GI7T. Accessed 15 August 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

