Retinoid signaling in skeletal development: Scoping the system for predictive toxicology
- PMID: 33202217
- PMCID: PMC11451096
- DOI: 10.1016/j.reprotox.2020.10.014
Retinoid signaling in skeletal development: Scoping the system for predictive toxicology
Abstract
All-trans retinoic acid (ATRA), the biologically active form of vitamin A, is instrumental in regulating the patterning and specification of the vertebrate embryo. Various animal models demonstrate adverse developmental phenotypes following experimental retinoid depletion or excess during pregnancy. Windows of vulnerability for altered skeletal patterning coincide with early specification of the body plan (gastrulation) and regional specification of precursor cell populations forming the facial skeleton (cranial neural crest), vertebral column (somites), and limbs (lateral plate mesoderm) during organogenesis. A common theme in physiological roles of ATRA signaling is mutual antagonism with FGF signaling. Consequences of genetic errors or environmental disruption of retinoid signaling include stage- and region-specific homeotic transformations to severe deficiencies for various skeletal elements. This review derives from an annex in Detailed Review Paper (DRP) of the OECD Test Guidelines Programme (Project 4.97) to support recommendations regarding assay development for the retinoid system and the use of resulting data in a regulatory context for developmental and reproductive toxicity (DART) testing.
Keywords: Developmental toxicity; Retinoid signaling; Skeletal development.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors report no declarations of interest.
Figures
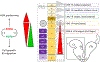

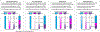
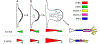

Similar articles
-
Revisiting the role of retinoid signaling in skeletal development.Birth Defects Res C Embryo Today. 2003 May;69(2):156-73. doi: 10.1002/bdrc.10010. Birth Defects Res C Embryo Today. 2003. PMID: 12955859 Review.
-
Retinoid signaling is required to complete the vertebrate cardiac left/right asymmetry pathway.Dev Biol. 2000 Jul 15;223(2):323-38. doi: 10.1006/dbio.2000.9754. Dev Biol. 2000. PMID: 10882519
-
Retinoid signalling is required for information transfer from mesoderm to neuroectoderm during gastrulation.Int J Dev Biol. 2010;54(4):599-608. doi: 10.1387/ijdb.082705fl. Int J Dev Biol. 2010. PMID: 20209433
-
Retinoid signalling and axial patterning during early vertebrate embryogenesis.Cell Mol Life Sci. 1997 Apr;53(4):339-49. doi: 10.1007/pl00000610. Cell Mol Life Sci. 1997. PMID: 9137625 Free PMC article. Review.
-
Retinoid signaling is required for chondrocyte maturation and endochondral bone formation during limb skeletogenesis.Dev Biol. 1999 Apr 15;208(2):375-91. doi: 10.1006/dbio.1999.9207. Dev Biol. 1999. PMID: 10191052
Cited by
-
Retinoic acid signaling and metabolism in heart failure.Am J Physiol Heart Circ Physiol. 2025 Apr 1;328(4):H792-H813. doi: 10.1152/ajpheart.00871.2024. Epub 2025 Feb 11. Am J Physiol Heart Circ Physiol. 2025. PMID: 39933792 Free PMC article. Review.
-
AOP Key Event Relationship report: Linking decreased retinoic acid levels with disrupted meiosis in developing oocytes.Curr Res Toxicol. 2022 Mar 18;3:100069. doi: 10.1016/j.crtox.2022.100069. eCollection 2022. Curr Res Toxicol. 2022. PMID: 35345548 Free PMC article.
-
Scientific opinion on the tolerable upper intake level for preformed vitamin A and β-carotene.EFSA J. 2024 Jun 6;22(6):e8814. doi: 10.2903/j.efsa.2024.8814. eCollection 2024 Jun. EFSA J. 2024. PMID: 38846679 Free PMC article.
-
Development and validation of CYP26A1 inhibition assay for high-throughput screening.Biotechnol J. 2024 Jun;19(6):e2300659. doi: 10.1002/biot.202300659. Biotechnol J. 2024. PMID: 38863121 Free PMC article.
-
Computational model for fetal skeletal defects potentially linked to disruption of retinoic acid signaling.Front Pharmacol. 2022 Sep 6;13:971296. doi: 10.3389/fphar.2022.971296. eCollection 2022. Front Pharmacol. 2022. PMID: 36172177 Free PMC article.
References
-
- Abe M, Maeda T and Wakisaka S (2008). “Retinoic acid affects craniofacial patterning by changing Fgf8 expression in the pharyngeal ectoderm.” Dev Growth Differ 50(9): 717–729. - PubMed
-
- Abu-Hijleh G and Padmanabhan R (1997). “Retinoic acid-induced abnormal development of hindlimb joints in the mouse.” Eur J Morphol 35(5): 327–336. - PubMed
-
- Ahir B, DeWoskin R, Baker N, Spencer R, Setzer R, Lau C and Knudsen T (2019). “Developmental toxicity Simulated in a dynamic virtual embryo model of early limb-bud outgrowth (in preparation).”
-
- Akimenko MA, Ekker M (1995). “ Anterior duplication of the Sonic hedgehog expression pattern in the pectoral fin buds of zebrafish treated with retinoic acid” Dev Biol 170 (1): 243–247 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

