CRISPR-Mediated Induction of Neuron-Enriched Mitochondrial Proteins Boosts Direct Glia-to-Neuron Conversion
- PMID: 33202244
- PMCID: PMC7939544
- DOI: 10.1016/j.stem.2020.10.015
CRISPR-Mediated Induction of Neuron-Enriched Mitochondrial Proteins Boosts Direct Glia-to-Neuron Conversion
Erratum in
-
CRISPR-Mediated Induction of Neuron-Enriched Mitochondrial Proteins Boosts Direct Glia-to-Neuron Conversion.Cell Stem Cell. 2021 Mar 4;28(3):584. doi: 10.1016/j.stem.2020.11.017. Cell Stem Cell. 2021. PMID: 33667363 Free PMC article. No abstract available.
Abstract
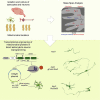
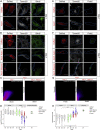
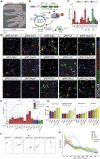
Astrocyte-to-neuron conversion is a promising avenue for neuronal replacement therapy. Neurons are particularly dependent on mitochondrial function, but how well mitochondria adapt to the new fate is unknown. Here, we determined the comprehensive mitochondrial proteome of cortical astrocytes and neurons, identifying about 150 significantly enriched mitochondrial proteins for each cell type, including transporters, metabolic enzymes, and cell-type-specific antioxidants. Monitoring their transition during reprogramming revealed late and only partial adaptation to the neuronal identity. Early dCas9-mediated activation of genes encoding mitochondrial proteins significantly improved conversion efficiency, particularly for neuron-enriched but not astrocyte-enriched antioxidant proteins. For example, Sod1 not only improves the survival of the converted neurons but also elicits a faster conversion pace, indicating that mitochondrial proteins act as enablers and drivers in this process. Transcriptional engineering of mitochondrial proteins with other functions improved reprogramming as well, demonstrating a broader role of mitochondrial proteins during fate conversion.
Keywords: CRISPR-a; antioxidant; direct reprogramming; metabolism; mitochondria; proteome.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


References
-
- Barker R.A., Götz M., Parmar M. New approaches for brain repair-from rescue to reprogramming. Nature. 2018;557:329–334. - PubMed
-
- Boulos S., Meloni B.P., Arthur P.G., Bojarski C., Knuckey N.W. Peroxiredoxin 2 overexpression protects cortical neuronal cultures from ischemic and oxidative injury but not glutamate excitotoxicity, whereas Cu/Zn superoxide dismutase 1 overexpression protects only against oxidative injury. J. Neurosci. Res. 2007;85:3089–3097. - PubMed
-
- Bresell A., Weinander R., Lundqvist G., Raza H., Shimoji M., Sun T.H., Balk L., Wiklund R., Eriksson J., Jansson C. Bioinformatic and enzymatic characterization of the MAPEG superfamily. FEBS J. 2005;272:1688–1703. - PubMed
-
- Breunig C.T., Neuner A.M., Giehrl-Schwab J., Wurst W., Götz M., Stricker S.H. A Customizable Protocol for String Assembly gRNA Cloning (STAgR) J. Vis. Exp. 2018;(142):e58556. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

