EBV-associated primary CNS lymphoma occurring after immunosuppression is a distinct immunobiological entity
- PMID: 33202420
- PMCID: PMC7976507
- DOI: 10.1182/blood.2020008520
EBV-associated primary CNS lymphoma occurring after immunosuppression is a distinct immunobiological entity
Abstract
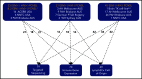
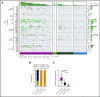
Primary central nervous system lymphoma (PCNSL) is confined to the brain, eyes, and cerebrospinal fluid without evidence of systemic spread. Rarely, PCNSL occurs in the context of immunosuppression (eg, posttransplant lymphoproliferative disorders or HIV [AIDS-related PCNSL]). These cases are poorly characterized, have dismal outcome, and are typically Epstein-Barr virus (EBV)-associated (ie, tissue-positive). We used targeted sequencing and digital multiplex gene expression to compare the genetic landscape and tumor microenvironment (TME) of 91 PCNSL tissues all with diffuse large B-cell lymphoma histology. Forty-seven were EBV tissue-negative: 45 EBV- HIV- PCNSL and 2 EBV- HIV+ PCNSL; and 44 were EBV tissue-positive: 23 EBV+ HIV+ PCNSL and 21 EBV+ HIV- PCNSL. As with prior studies, EBV- HIV- PCNSL had frequent MYD88, CD79B, and PIM1 mutations, and enrichment for the activated B-cell (ABC) cell-of-origin subtype. In contrast, these mutations were absent in all EBV tissue-positive cases and ABC frequency was low. Furthermore, copy number loss in HLA class I/II and antigen-presenting/processing genes were rarely observed, indicating retained antigen presentation. To counter this, EBV+ HIV- PCNSL had a tolerogenic TME with elevated macrophage and immune-checkpoint gene expression, whereas AIDS-related PCNSL had low CD4 gene counts. EBV-associated PCNSL in the immunosuppressed is immunobiologically distinct from EBV- HIV- PCNSL, and, despite expressing an immunogenic virus, retains the ability to present EBV antigens. Results provide a framework for targeted treatment.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





Comment in
-
Sorting biologic subtypes of primary CNS lymphoma.Blood. 2021 Mar 18;137(11):1436-1437. doi: 10.1182/blood.2020009968. Blood. 2021. PMID: 33734333 Free PMC article.
References
-
- Nakamura T, Tateishi K, Niwa T, et al. . Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol. 2016;42(3):279-290. - PubMed
-
- Gonzalez-Aguilar A, Idbaih A, Boisselier B, et al. . Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res. 2012;18(19):5203-5211. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

