Efficacy and Toxicity of Different Chemotherapy Protocols for Concurrent Chemoradiation in Non-Small Cell Lung Cancer-A Secondary Analysis of the PET Plan Trial
- PMID: 33202825
- PMCID: PMC7697287
- DOI: 10.3390/cancers12113359
Efficacy and Toxicity of Different Chemotherapy Protocols for Concurrent Chemoradiation in Non-Small Cell Lung Cancer-A Secondary Analysis of the PET Plan Trial
Abstract
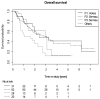
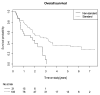
(1) Background: The optimal chemotherapy (CHT) regimen for concurrent chemoradiation (cCRT) is not well defined. In this secondary analysis of the international randomized PET-Plan trial, we evaluate the efficacy of different CHT. (2) Methods: Patients with inoperable NSCLC were randomized at a 1:1 ratio regarding the target volume definition and received isotoxically dose-escalated cCRT using cisplatin 80 mg/m2 (day 1, 22) and vinorelbin 15 mg/m2 (day 1, 8, 22, 29) (P1) or cisplatin 20 mg/m2 (day 1-5, 29-33) and vinorelbin 12.5 mg/m2 (day 1, 8, 15, 29, 36, 43) (P2) or carboplatin AUC1 (day 1-5, 29-33) and vinorelbin 12.5 mg/m2 (day 1, 8, 15, 29, 36, 43) (P3) or other CHT at the treating physician's discretion. (3) Results: Between 05/2009 and 11/2016, 205 patients were randomized and 172 included in the per-protocol analysis. Patients treated in P1 or P2 had a better overall survival (OS) compared to P3 (p = 0.015, p = 0.01, respectively). Patients treated with carboplatin had a worse OS compared to cisplatin (HR 1.78, p = 0.03), but the difference did not remain significant after adjusting for age, ECOG, cardiac function creatinine and completeness of CHT. (4) Conclusions: Carboplatin doublets show no significant difference compared to cisplatin, after adjusting for possibly relevant factors, probably due to existing selection bias.
Keywords: NSCLC; chemoradiotherapy; concomitant chemoradiation; non-small cell lung cancer.
Conflict of interest statement
SA receives fees from the German cancer consortium, CW received fees from Mylan Inc. Alvotech, Roche, AstraZeneca, Boehringer Ingelheim, BMS, Chugai, Pfizer, Takeda, IPSEN, Lilly. All other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Ettinger D.S., Wood D.E., Aggarwal C., Aisner D.L., Akerley W., Bauman J.R., Bharat A., Bruno D.S., Chang J.Y., Chirieac L.R., et al. NCCN guidelines insights: Non-small cell lung cancer, version 1.2020. J. Natl. Compr. Cancer Netw. JNCCN. 2019;17:1464–1472. doi: 10.6004/jnccn.2019.0059. - DOI - PubMed
-
- Yamamoto N., Nakagawa K., Nishimura Y., Tsujino K., Satouchi M., Kudo S., Hida T., Kawahara M., Takeda K., Katakami N., et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010;28:3739–3745. doi: 10.1200/JCO.2009.24.5050. - DOI - PubMed
-
- Segawa Y., Kiura K., Takigawa N., Kamei H., Harita S., Hiraki S., Watanabe Y., Sugimoto K., Shibayama T., Yonei T., et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010;28:3299–3306. doi: 10.1200/JCO.2009.24.7577. - DOI - PubMed
-
- Liu T., He Z., Dang J., Li G. Comparative efficacy and safety for different chemotherapy regimens used concurrently with thoracic radiation for locally advanced non-small cell lung cancer: A systematic review and network meta-analysis. Radiat. Oncol. 2019;14:55. doi: 10.1186/s13014-019-1239-7. - DOI - PMC - PubMed
-
- Senan S., Brade A., Wang L.H., Vansteenkiste J., Dakhil S., Biesma B., Martinez Aguillo M., Aerts J., Govindan R., Rubio-Viqueira B., et al. PROCLAIM: Randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016;34:953–962. doi: 10.1200/JCO.2015.64.8824. - DOI - PubMed
LinkOut - more resources
Full Text Sources