Electrochemical Immunosensors Based on Screen-Printed Gold and Glassy Carbon Electrodes: Comparison of Performance for Respiratory Syncytial Virus Detection
- PMID: 33202922
- PMCID: PMC7698328
- DOI: 10.3390/bios10110175
Electrochemical Immunosensors Based on Screen-Printed Gold and Glassy Carbon Electrodes: Comparison of Performance for Respiratory Syncytial Virus Detection
Abstract
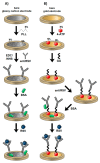
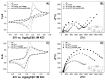
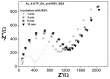
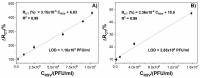
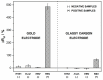
This paper presents the development and comparison of label-free electrochemical immunosensors based on screen-printed gold and glassy carbon (GC) disc electrodes for efficient and rapid detection of respiratory syncytial virus (RSV). Briefly, the antibody specific to the F protein of RSV was successfully immobilized on modified electrodes. Antibody coupling on the Au surface was conducted via 4-aminothiophenol (4-ATP) and glutaraldehyde (GA). The GC surface was modified with poly-L-lysine (PLL) for direct anti-RSV conjugation after EDC/NHS (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-Hydroxysuccinimide) activation. Electrochemical characterizations of the immunosensors were carried out by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). GC-based immunosensors show a dynamic range of antigen detection from 1.0 × 105 PFU/mL to 1.5×107 PFU/mL, more than 1.0 × 105 PFU/mL to 1.0 × 107 PFU/mL for the Au-based sensor. However, the GC platform is less sensitive and shows a higher detection limit (LOD) for RSV. The limit of detection of the Au immunosensor is 1.1 × 103 PFU/mL, three orders of magnitude lower than 2.85 × 106 PFU/mL for GC. Thus, the Au-based immunosensor has better analytical performance for virus detection than a carbon-based platform due to high sensitivity and very low RSV detection, obtained with good reproducibility.
Keywords: cyclic voltammetry; electrochemical impedance spectroscopy; glassy carbon; gold electrode; respiratory syncytial virus; sensor.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Resorc[4]arene-Modified Gold-Decorated Magnetic Nanoparticles for Immunosensor Development.Bioconjug Chem. 2023 Mar 15;34(3):529-537. doi: 10.1021/acs.bioconjchem.2c00605. Epub 2023 Feb 8. Bioconjug Chem. 2023. PMID: 36753752 Free PMC article.
-
A label-free electrochemical biosensor based on tubulin immobilized on gold nanoparticle/glassy carbon electrode for the determination of vinblastine.Anal Bioanal Chem. 2017 Sep;409(22):5269-5278. doi: 10.1007/s00216-017-0471-y. Epub 2017 Jun 30. Anal Bioanal Chem. 2017. PMID: 28667386
-
Rapid quantitative detection of Brucella melitensis by a label-free impedance immunosensor based on a gold nanoparticle-modified screen-printed carbon electrode.Sensors (Basel). 2013 Jul 4;13(7):8551-63. doi: 10.3390/s130708551. Sensors (Basel). 2013. PMID: 23881126 Free PMC article.
-
Cost-Effective and Handmade Paper-Based Immunosensing Device for Electrochemical Detection of Influenza Virus.Sensors (Basel). 2017 Nov 11;17(11):2597. doi: 10.3390/s17112597. Sensors (Basel). 2017. PMID: 29137115 Free PMC article.
-
Fluorescence immunosensor based on functional nanomaterials and its application in tumor biomarker detection.RSC Adv. 2022 Nov 1;12(48):31369-31379. doi: 10.1039/d2ra04989a. eCollection 2022 Oct 27. RSC Adv. 2022. PMID: 36349017 Free PMC article. Review.
Cited by
-
An Ultrasensitive Biosensor for Detection of Femtogram Levels of the Cancer Antigen AGR2 Using Monoclonal Antibody Modified Screen-Printed Gold Electrodes.Biosensors (Basel). 2021 Jun 7;11(6):184. doi: 10.3390/bios11060184. Biosensors (Basel). 2021. PMID: 34200338 Free PMC article.
-
Novel Cytochrome P450-3A4 Enzymatic Nanobiosensor for Lapatinib (a Breast Cancer Drug) Developed on a Poly(anilino-co-4-aminobenzoic Acid-Green-Synthesised Indium Nanoparticle) Platform.Biosensors (Basel). 2023 Sep 21;13(9):897. doi: 10.3390/bios13090897. Biosensors (Basel). 2023. PMID: 37754131 Free PMC article.
-
Diagnostic techniques for critical respiratory infections: Update on current methods.Heliyon. 2023 Aug 5;9(8):e18957. doi: 10.1016/j.heliyon.2023.e18957. eCollection 2023 Aug. Heliyon. 2023. PMID: 37600408 Free PMC article. Review.
-
Pathogen detection with electrochemical biosensors: Advantages, challenges and future perspectives.J Electroanal Chem (Lausanne). 2021 Feb 1;882:114989. doi: 10.1016/j.jelechem.2021.114989. Epub 2021 Jan 9. J Electroanal Chem (Lausanne). 2021. PMID: 33456428 Free PMC article. Review.
-
Impact of nanotechnology on conventional and artificial intelligence-based biosensing strategies for the detection of viruses.Discov Nano. 2023 Apr 1;18(1):58. doi: 10.1186/s11671-023-03842-4. eCollection 2023 Dec. Discov Nano. 2023. PMID: 37032711 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

