Anti-Influenza Effect of Nanosilver in a Mouse Model
- PMID: 33202939
- PMCID: PMC7712555
- DOI: 10.3390/vaccines8040679
Anti-Influenza Effect of Nanosilver in a Mouse Model
Abstract
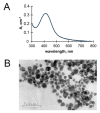
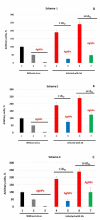
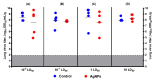
The present study assesses copper metabolism of the host organism as a target of antiviral strategy, basing on the "virocell" concept. Silver nanoparticles (AgNPs) were used as a specific active agent because they reduce the level of holo-ceruloplasmin, the main extracellular cuproenzyme. The mouse model of influenza virus A infection was used with two doses: 1 LD50 and 10 LD50. Three treatment regimens were used: Scheme 1-mice were pretreated 4 days before infection and then every day during infection development; Scheme 2-mice were pretreated four days before infection and on the day of virus infection; Scheme 3-virus infection and AgNP treatment started simultaneously, and mice were injected with AgNPs until the end of the experiment. The mice treated by Scheme 1 demonstrated significantly lower mortality, the protection index reached 60-70% at the end of the experiment, and mean lifespan was prolonged. In addition, the treatment of the animals with AgNPs resulted in normalization of the weight dynamics. Despite the amelioration of the infection, AgNP treatment did not influence influenza virus replication. The possibility of using nanosilver as an effective indirectly-acting antiviral drug is discussed.
Keywords: ceruloplasmin; copper status; indirectly-acting antiviral drug; influenza; influenza virus replication; prophylaxis and treatment; silver nanoparticles.
Conflict of interest statement
The author declares no conflict of interest.
Figures





References
-
- CDC Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2019–2020 Influenza Season. [(accessed on 5 October 2020)]; Available online: https://www.cdc.gov/mmwr/volumes/68/rr/rr6803a1.htm.
-
- WHO Influenza Vaccines. [(accessed on 23 October 2020)]; Available online: https://www.who.int/influenza/vaccines/en/
LinkOut - more resources
Full Text Sources
Research Materials

