Glutathione Enhances Auxin Sensitivity in Arabidopsis Roots
- PMID: 33202956
- PMCID: PMC7697393
- DOI: 10.3390/biom10111550
Glutathione Enhances Auxin Sensitivity in Arabidopsis Roots
Abstract
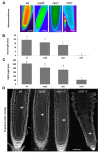
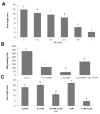
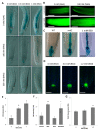
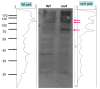
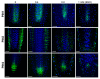
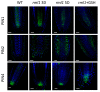
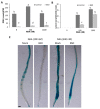
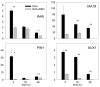
Root development is regulated by the tripeptide glutathione (GSH), a strong non-enzymatic antioxidant found in plants but with a poorly understood function in roots. Here, Arabidopsis mutants deficient in GSH biosynthesis (cad2, rax1, and rml1) and plants treated with the GSH biosynthesis inhibitor buthionine sulfoximine (BSO) showed root growth inhibition, significant alterations in the root apical meristem (RAM) structure (length and cell division), and defects in lateral root formation. Investigation of the molecular mechanisms of GSH action showed that GSH deficiency modulated total ubiquitination of proteins and inhibited the auxin-related, ubiquitination-dependent degradation of Aux/IAA proteins and the transcriptional activation of early auxin-responsive genes. However, the DR5 auxin transcriptional response differed in root apical meristem (RAM) and pericycle cells. The RAM DR5 signal was increased due to the up-regulation of the auxin biosynthesis TAA1 protein and down-regulation of PIN4 and PIN2, which can act as auxin sinks in the root tip. The transcription auxin response (the DR5 signal and expression of auxin responsive genes) in isolated roots, induced by a low (0.1 µM) auxin concentration, was blocked following GSH depletion of the roots by BSO treatment. A higher auxin concentration (0.5 µM) offset this GSH deficiency effect on DR5 expression, indicating that GSH deficiency does not completely block the transcriptional auxin response, but decreases its sensitivity. The ROS regulation of GSH, the active GSH role in cell proliferation, and GSH cross-talk with auxin assume a potential role for GSH in the modulation of root architecture under stress conditions.
Keywords: auxin; auxin biosynthesis; auxin response; auxin transport; glutathne (GSH); lateral root formation; root apical meristem.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

