An Immediate and Long-Term Complication of COVID-19 May Be Type 2 Diabetes Mellitus: The Central Role of β-Cell Dysfunction, Apoptosis and Exploration of Possible Mechanisms
- PMID: 33202960
- PMCID: PMC7697826
- DOI: 10.3390/cells9112475
An Immediate and Long-Term Complication of COVID-19 May Be Type 2 Diabetes Mellitus: The Central Role of β-Cell Dysfunction, Apoptosis and Exploration of Possible Mechanisms
Abstract
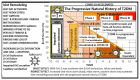
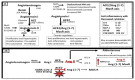
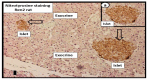
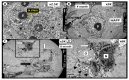
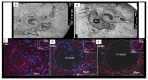
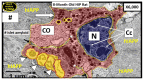
The novel coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was declared a pandemic by the WHO on 19 March 2020. This pandemic is associated with markedly elevated blood glucose levels and a remarkable degree of insulin resistance, which suggests pancreatic islet β-cell dysfunction or apoptosis and insulin's inability to dispose of glucose into cellular tissues. Diabetes is known to be one of the top pre-existing co-morbidities associated with the severity of COVID-19 along with hypertension, cardiocerebrovascular disease, advanced age, male gender, and recently obesity. This review focuses on how COVID-19 may be responsible for the accelerated development of type 2 diabetes mellitus (T2DM) as one of its acute and suspected long-term complications. These observations implicate an active role of metabolic syndrome, systemic and tissue islet renin-angiotensin-aldosterone system, redox stress, inflammation, islet fibrosis, amyloid deposition along with β-cell dysfunction and apoptosis in those who develop T2DM. Utilizing light and electron microscopy in preclinical rodent models and human islets may help to better understand how COVID-19 accelerates islet and β-cell injury and remodeling to result in the long-term complications of T2DM.
Keywords: ACE2; SARS-CoV-2; amylin; fibrosis; islet; islet amyloid; metabolic syndrome; oxidative stress; renin–angiotensin–aldosterone-system; β-cell apoptosis.
Conflict of interest statement
The author declares that there is no conflict of interest.
Figures










Similar articles
-
Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19).J Pathol. 2020 Jul;251(3):228-248. doi: 10.1002/path.5471. Epub 2020 Jun 10. J Pathol. 2020. PMID: 32418199 Free PMC article. Review.
-
COVID-19, Renin-Angiotensin System and Endothelial Dysfunction.Cells. 2020 Jul 9;9(7):1652. doi: 10.3390/cells9071652. Cells. 2020. PMID: 32660065 Free PMC article. Review.
-
Endothelial activation and dysfunction in metabolic syndrome, type 2 diabetes and coronavirus disease 2019.J Int Med Res. 2020 Jul;48(7):300060520939746. doi: 10.1177/0300060520939746. J Int Med Res. 2020. PMID: 32722979 Free PMC article. Review.
-
Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2.Circ Res. 2020 May 8;126(10):1456-1474. doi: 10.1161/CIRCRESAHA.120.317015. Epub 2020 Apr 8. Circ Res. 2020. PMID: 32264791 Free PMC article. Review.
-
Genetic Hypothesis and Pharmacogenetics Side of Renin-Angiotensin-System in COVID-19.Genes (Basel). 2020 Sep 3;11(9):1044. doi: 10.3390/genes11091044. Genes (Basel). 2020. PMID: 32899439 Free PMC article.
Cited by
-
Viral infiltration of pancreatic islets in patients with COVID-19.Nat Commun. 2021 Jun 10;12(1):3534. doi: 10.1038/s41467-021-23886-3. Nat Commun. 2021. PMID: 34112801 Free PMC article.
-
Post-acute COVID-19 syndrome in patients after 12 months from COVID-19 infection in Korea.BMC Infect Dis. 2022 Jan 27;22(1):93. doi: 10.1186/s12879-022-07062-6. BMC Infect Dis. 2022. PMID: 35086489 Free PMC article.
-
Effect of a SARS-CoV-2 Protein Fragment on the Amyloidogenic Propensity of Human Islet Amyloid Polypeptide.ACS Chem Neurosci. 2024 Dec 18;15(24):4431-4440. doi: 10.1021/acschemneuro.4c00473. Epub 2024 Nov 24. ACS Chem Neurosci. 2024. PMID: 39582236 Free PMC article.
-
The Nursing Effect of Individualized Management on Patients With Diabetes Mellitus Type 2 and Hypertension.Front Endocrinol (Lausanne). 2022 Mar 17;13:846419. doi: 10.3389/fendo.2022.846419. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35370933 Free PMC article. Clinical Trial.
-
Apoptosis in Type 2 Diabetes: Can It Be Prevented? Hippo Pathway Prospects.Int J Mol Sci. 2022 Jan 7;23(2):636. doi: 10.3390/ijms23020636. Int J Mol Sci. 2022. PMID: 35054822 Free PMC article. Review.
References
-
- Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., Amadou C., Arnault G., Baudoux F., Bauduceau B., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

