From Target-Oriented to Motif-Oriented: A Case Study on Nannocystin Total Synthesis
- PMID: 33203102
- PMCID: PMC7697126
- DOI: 10.3390/molecules25225327
From Target-Oriented to Motif-Oriented: A Case Study on Nannocystin Total Synthesis
Abstract
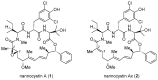
Natural product total synthesis is in essence target-oriented in that a set of organic transformations are orchestrated into a workable process, leading ultimately to the target molecule with a predefined architecture. For a bioactive lead, proof of synthetic viability is merely the beginning. Ensuing effort repurposes the initial synthesis for structural diversification in order to probe structure-activity relationship (SAR). Yet accessibility is not equal to flexibility; moving from convergency to divergency, it is not always feasible to explore the chemical space around a particular substructure of interest simply by tweaking an established route. In this situation, the motif-oriented strategy becomes a superior choice, which gives priority to synthetic flexibility at the concerned site such that a route is adopted only if it is capable of implementing diversification therein. This strategy was recently devised by Fürstner et al., enabling them to achieve total synthesis of both natural and non-natural nannocystins varied at an otherwise challenging position. The present review examines seven distinctive nannocystin total syntheses reported thus far and showcases the merits of conventional (target-oriented) as well as motif-oriented strategies, concluding that these two approaches complement each other and are both indispensable for natural product based drug discovery.
Keywords: macrocyclization; motif-oriented; nannocystin; total synthesis.
Conflict of interest statement
The author declares no conflict of interest.
Figures







References
-
- Hoffmann H., Kogler H., Heyse W., Matter H., Caspers M., Schummer D., Klemke-Jahn C., Bauer A., Penarier G., Debussche L., et al. Discovery, Structure Elucidation, and Biological Characterization of Nannocystin A, a Macrocyclic Myxobacterial Metabolite with Potent Antiproliferative Properties. Angew. Chem. Int. Ed. 2015;54:10145–10148. doi: 10.1002/anie.201411377. - DOI - PubMed
-
- Krastel P., Roggo S., Schirle M., Ross N.T., Perruccio F., Aspesi P., Aust T., Buntin K., Estoppey D., Liechty B., et al. Nannocystin A: An Elongation Factor 1 Inhibitor from Myxobacteria with Differential Anti-Cancer Properties. Angew. Chem. Int. Ed. 2015;54:10149–10154. doi: 10.1002/anie.201505069. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

