Sodium para-aminosalicylic acid inhibits manganese-induced NLRP3 inflammasome-dependent pyroptosis by inhibiting NF-κB pathway activation and oxidative stress
- PMID: 33203418
- PMCID: PMC7670624
- DOI: 10.1186/s12974-020-02018-6
Sodium para-aminosalicylic acid inhibits manganese-induced NLRP3 inflammasome-dependent pyroptosis by inhibiting NF-κB pathway activation and oxidative stress
Abstract
Background: The activation of NOD-like receptor protein 3 (NLRP3) inflammasome-dependent pyroptosis has been shown to play a vital role in the pathology of manganese (Mn)-induced neurotoxicity. Sodium para-aminosalicylic acid (PAS-Na) has a positive effect on the treatment of manganism. However, the mechanism is still unclear. We hypothesized that PAS-Na might act through NLRP3.
Methods: The microglial cell line BV2 and male Sprague-Dawley rats were used to investigate the impacts of PAS-Na on Mn-induced NLRP3 inflammasome-dependent pyroptosis. The related protein of the NF-κB pathway and NLRP3-inflammasome-dependent pyroptosis was detected by western blot. The reactive oxygen species and mitochondrial membrane potential were detected by immunofluorescence staining and flow cytometry. The activation of microglia and the gasdermin D (GSDMD) were detected by immunofluorescence staining.
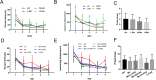
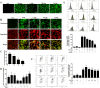
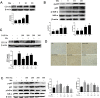
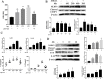
Results: Our results showed that Mn treatment induced oxidative stress and activated the NF-κB pathway by increasing the phosphorylation of p65 and IkB-α in BV2 cells and in the basal ganglia of rats. PAS-Na could alleviate Mn-induced oxidative stress damage by inhibiting ROS generation, increasing mitochondrial membrane potential and ATP levels, thereby reducing the phosphorylation of p65 and IkB-α. Besides, Mn treatment could activate the NLRP3 pathway and promote the secretion of IL-18 and IL-1β, mediating pyroptosis in BV2 cells and in the basal ganglia and hippocampus of rats. But an inhibitor of NF-κb (JSH-23) treatment could significantly reduce LDH release, the expression of NLRP3 and Cleaved CASP1 protein and IL-1β and IL-18 mRNA level in BV2 cells. Interestingly, the effect of PAS-Na treatment in Mn-treated BV2 cells is similar to those of JSH-23. Besides, immunofluorescence results showed that PAS-Na reduced the increase number of activated microglia, which stained positively for GSDMD.
Conclusion: PAS-Na antagonized Mn-induced NLRP3 inflammasome dependent pyroptosis through inhibiting NF-κB pathway activation and oxidative stress.
Keywords: Mn; NF-κB pathway; NLRP3 inflammasome; Oxidative stress; PAS-Na; pyroptosis.
Conflict of interest statement
There is no conflict of interest with any of the authors. This manuscript has not been published before and is not currently submitted for review to any other journal.
Figures


References
-
- Jiang YM, Mo XA, Du FQ, Fu X, Zhu XY, Gao HY, Xie JL, Liao FL, Pira E, Zheng W. Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. J Occup Environ Med. 2006;48:644–649. doi: 10.1097/01.jom.0000204114.01893.3e. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

