The RESPECT study: a feasibility randomised controlled trial of a sexual health promotion intervention for people with serious mental illness in community mental health services in the UK
- PMID: 33203433
- PMCID: PMC7673083
- DOI: 10.1186/s12889-020-09661-x
The RESPECT study: a feasibility randomised controlled trial of a sexual health promotion intervention for people with serious mental illness in community mental health services in the UK
Abstract
Background: People with serious mental illness (SMI) have sexual health needs but there is little evidence to inform effective interventions to address them. In fact, there are few studies that have addressed this topic for people with SMI outside USA and Brazil. Therefore, the aim of the study was to establish the acceptability and feasibility of a trial of a sexual health promotion intervention for people with SMI in the UK.
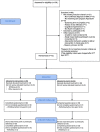
Method: The RESPECT study was a two-armed randomised controlled, open feasibility trial (RCT) comparing Sexual health promotion intervention (3 individual sessions of 1 h) (I) or treatment as usual (TAU) for adults aged 18 or over, with SMI, within community mental health services in four UK cities. The main outcome of interest was the percentage who consented to participate, and retained in each arm of the trial, retention for the intervention, and completeness of data collection. A nested qualitative study obtained the views of participants regarding the acceptability of the study using individual telephone interviews conducted by lived experience researchers.
Results: Of a target sample of 100, a total of 72 people were enrolled in the trial over 12 months. Recruitment in the initial months was low and so an extension was granted. However this extension meant that the later recruited participants would only be followed up to the 3 month point. There was good retention in the intervention and the study as a whole; 77.8% of those allocated to intervention (n = 28) received it. At three months, 81.9% (30 I; 29 TAU) and at 6 months, 76.3% (13 I and 16 TAU) completed the follow-up data collection. No adverse events were reported. There was good completeness of the data. The sexual health outcomes for the intervention group changed in favour of the intervention. Based on analysis of the qualitative interviews, the methods of recruitment, the quality of the participant information, the data collection, and the intervention were deemed to be acceptable to the participants (n = 22).
Conclusions: The target of 100 participants was not achieved within the study's timescale. However, effective strategies were identified that improved recruitment in the final few months. Retention rates and completeness of data in both groups indicate that it is acceptable and feasible to undertake a study promoting sexual health for people with SMI. A fully powered RCT is required to establish effectiveness of the intervention in adoption of safer sex.
Study registration: ISRCTN Registry ISRCTN15747739 prospectively registered 5th July 2016.
Keywords: Feasibility; Mental health; Psychosis; Randomised controlled trial; Sexual behavior; Sexual health.
Conflict of interest statement
Hughes has received honoraria from Lundbeck pharmaceuticals for delivery of educational talks to mental health staff on the topic of sexual health promotion in mental health.
Similar articles
-
Sexual health promotion in people with severe mental illness: the RESPECT feasibility RCT.Health Technol Assess. 2019 Dec;23(65):1-136. doi: 10.3310/hta23650. Health Technol Assess. 2019. PMID: 31854292 Free PMC article. Clinical Trial.
-
Psychological intervention, antipsychotic medication or a combined treatment for adolescents with a first episode of psychosis: the MAPS feasibility three-arm RCT.Health Technol Assess. 2021 Jan;25(4):1-124. doi: 10.3310/hta25040. Health Technol Assess. 2021. PMID: 33496261 Free PMC article. Clinical Trial.
-
An intervention to improve the quality of life in children of parents with serious mental illness: the Young SMILES feasibility RCT.Health Technol Assess. 2020 Nov;24(59):1-136. doi: 10.3310/hta24590. Health Technol Assess. 2020. PMID: 33196410 Free PMC article. Clinical Trial.
-
Development and evaluation of a de-escalation training intervention in adult acute and forensic units: the EDITION systematic review and feasibility trial.Health Technol Assess. 2024 Jan;28(3):1-120. doi: 10.3310/FGGW6874. Health Technol Assess. 2024. PMID: 38343036 Free PMC article.
-
Preventing blood-borne virus infection in people who inject drugs in the UK: systematic review, stakeholder interviews, psychosocial intervention development and feasibility randomised controlled trial.Health Technol Assess. 2017 Nov;21(72):1-312. doi: 10.3310/hta21720. Health Technol Assess. 2017. PMID: 29208190 Free PMC article. Clinical Trial.
Cited by
-
Sex education for patients with severe mental illness in Iran: A qualitative study.PEC Innov. 2022 Jan 5;1:100016. doi: 10.1016/j.pecinn.2022.100016. eCollection 2022 Dec. PEC Innov. 2022. PMID: 37213718 Free PMC article.
-
A peer-led learning program about intimate and romantic relationships for persons with mental disorders (AIRIKI): co-creation pilot feasibility study.BMC Psychiatry. 2023 Oct 19;23(1):767. doi: 10.1186/s12888-023-05254-1. BMC Psychiatry. 2023. PMID: 37858119 Free PMC article. Clinical Trial.
-
Breaking down barriers to conversations about sexual and reproductive health.World Psychiatry. 2025 Jun;24(2):220-221. doi: 10.1002/wps.21309. World Psychiatry. 2025. PMID: 40371802 Free PMC article. No abstract available.
-
Evaluating family knowledge about sexual health in patients with severe mental illness: a qualitative study in Iran.BMC Psychiatry. 2022 Mar 10;22(1):174. doi: 10.1186/s12888-022-03788-4. BMC Psychiatry. 2022. PMID: 35272647 Free PMC article.
References
-
- Simoila L, Isometsä E, Gissler M, Suvisaari J, Sailas E, Halmesmäki E, Lindberg N. Schizophrenia and induced abortions: A national register-based follow-up study among Finnish women born between 1965–1980 with schizophrenia or schizoaffective disorder. Schizophr Res. 2018;192:142–147. doi: 10.1016/j.schres.2017.05.039. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical