Epigenetic profiling of Italian patients identified methylation sites associated with hereditary transthyretin amyloidosis
- PMID: 33203445
- PMCID: PMC7672937
- DOI: 10.1186/s13148-020-00967-6
Epigenetic profiling of Italian patients identified methylation sites associated with hereditary transthyretin amyloidosis
Abstract
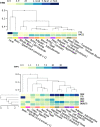
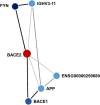
Hereditary transthyretin (TTR) amyloidosis (hATTR) is a rare life-threatening disorder caused by amyloidogenic coding mutations located in TTR gene. To understand the high phenotypic variability observed among carriers of TTR disease-causing mutations, we conducted an epigenome-wide association study (EWAS) assessing more than 700,000 methylation sites and testing epigenetic difference of TTR coding mutation carriers vs. non-carriers. We observed a significant methylation change at cg09097335 site located in Beta-secretase 2 (BACE2) gene (standardized regression coefficient = -0.60, p = 6.26 × 10-8). This gene is involved in a protein interaction network enriched for biological processes and molecular pathways related to amyloid-beta metabolism (Gene Ontology: 0050435, q = 0.007), amyloid fiber formation (Reactome HSA-977225, q = 0.008), and Alzheimer's disease (KEGG hsa05010, q = 2.2 × 10-4). Additionally, TTR and BACE2 share APP (amyloid-beta precursor protein) as a validated protein interactor. Within TTR gene region, we observed that Val30Met disrupts a methylation site, cg13139646, causing a drastic hypomethylation in carriers of this amyloidogenic mutation (standardized regression coefficient = -2.18, p = 3.34 × 10-11). Cg13139646 showed co-methylation with cg19203115 (Pearson's r2 = 0.32), which showed significant epigenetic differences between symptomatic and asymptomatic carriers of amyloidogenic mutations (standardized regression coefficient = -0.56, p = 8.6 × 10-4). In conclusion, we provide novel insights related to the molecular mechanisms involved in the complex heterogeneity of hATTR, highlighting the role of epigenetic regulation in this rare disorder.
Keywords: Amyloidosis; Epigenetics; Methylation; Modifier gene; Val30Met mutation; hATTR.
Conflict of interest statement
Drs. Fuciarelli and Polimanti received research grants from Pfizer Inc. to conduct epigenetic studies of hATTR. The other authors reported no biomedical financial interests or potential conflicts of interest.
Figures

References
-
- Parman Y, Adams D, Obici L, Galan L, Guergueltcheva V, Suhr OB, et al. Sixty years of transthyretin familial amyloid polyneuropathy (TTR-FAP) in Europe: where are we now? A European network approach to defining the epidemiology and management patterns for TTR-FAP. Curr Opin Neurol. 2016;29(Suppl 1):S3–S13. doi: 10.1097/WCO.0000000000000288. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

