NF-κB activation persists into the remodeling phase of tendon healing and promotes myofibroblast survival
- PMID: 33203721
- PMCID: PMC7717665
- DOI: 10.1126/scisignal.abb7209
NF-κB activation persists into the remodeling phase of tendon healing and promotes myofibroblast survival
Abstract
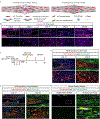
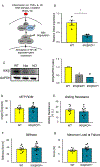
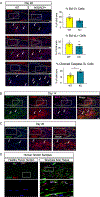
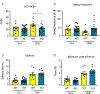
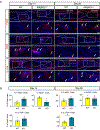
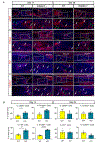
Although inflammation is necessary during the early phases of tissue repair, persistent inflammation contributes to fibrosis. Acute tendon injuries often heal through a fibrotic mechanism, which impedes regeneration and functional recovery. Because inflammation mediated by nuclear factor κB (NF-κB) signaling is implicated in this process, we examined the spatial, temporal, and cell type-specific activation profile of canonical NF-κB signaling during tendon healing. NF-κB signaling was maintained through all phases of tendon healing in mice, including the remodeling phase, and tenocytes and myofibroblasts from the Scleraxis (Scx) lineage were the predominant populations that retained NF-κB activation into the late stages of repair. We confirmed persistent NF-κB activation in myofibroblasts in human tendon scar tissue. Deleting the canonical NF-κB kinase, IKKβ, in Scx-lineage cells in mice increased apoptosis and the deposition of the matrix protein periostin during the late stages of tendon repair, suggesting that persistent NF-κB signaling may facilitate myofibroblast survival and fibrotic progression. Consistent with this, myofibroblasts in human tendon scar samples displayed enhanced prosurvival signaling compared to control tissue. Together, these data suggest that NF-κB may contribute to fibrotic tendon healing through both inflammation-dependent and inflammation-independent functions, such as NF-κB-mediated cell survival.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures



Similar articles
-
Deletion of NFKB1 enhances canonical NF-κB signaling and increases macrophage and myofibroblast content during tendon healing.Sci Rep. 2019 Jul 29;9(1):10926. doi: 10.1038/s41598-019-47461-5. Sci Rep. 2019. PMID: 31358843 Free PMC article.
-
Transcription factor scleraxis vitally contributes to progenitor lineage direction in wound healing of adult tendon in mice.J Biol Chem. 2018 Apr 20;293(16):5766-5780. doi: 10.1074/jbc.RA118.001987. Epub 2018 Mar 5. J Biol Chem. 2018. PMID: 29507095 Free PMC article.
-
Tendon healing in vitro: activation of NIK, IKKalpha, IKKbeta, and NF- kappaB genes in signal pathway and proliferation of tenocytes.Plast Reconstr Surg. 2004 May;113(6):1703-11. doi: 10.1097/01.prs.0000117365.48381.42. Plast Reconstr Surg. 2004. PMID: 15114132
-
Molecular dissection of tendon development and healing: Insights into tenogenic phenotypes and functions.J Biol Chem. 2025 Apr;301(4):108353. doi: 10.1016/j.jbc.2025.108353. Epub 2025 Feb 25. J Biol Chem. 2025. PMID: 40015639 Free PMC article. Review.
-
The roles and mechanisms of the NF-κB signaling pathway in tendon disorders.Front Vet Sci. 2024 Jun 24;11:1382239. doi: 10.3389/fvets.2024.1382239. eCollection 2024. Front Vet Sci. 2024. PMID: 38978635 Free PMC article. Review.
Cited by
-
A mineralizing pool of Gli1-expressing progenitors builds the tendon enthesis and demonstrates therapeutic potential.Cell Stem Cell. 2022 Dec 1;29(12):1669-1684.e6. doi: 10.1016/j.stem.2022.11.007. Cell Stem Cell. 2022. PMID: 36459968 Free PMC article.
-
Nonsurgical treatment reduces tendon inflammation and elevates tendon markers in early healing.J Orthop Res. 2022 Oct;40(10):2308-2319. doi: 10.1002/jor.25251. Epub 2022 Jan 7. J Orthop Res. 2022. PMID: 34935170 Free PMC article.
-
Understanding Fibrous Tissue in the Effective Healing of Rotator Cuff Injury.J Surg Res (Houst). 2024;7(2):215-228. doi: 10.26502/jsr.10020363. Epub 2024 May 21. J Surg Res (Houst). 2024. PMID: 38872898 Free PMC article.
-
Depletion of Scleraxis-lineage cells during tendon healing transiently impairs multi-scale restoration of tendon structure during early healing.PLoS One. 2022 Oct 14;17(10):e0274227. doi: 10.1371/journal.pone.0274227. eCollection 2022. PLoS One. 2022. PMID: 36240193 Free PMC article.
-
Magnesium ions attenuate tendon graft fibrosis during its ligamentization after ACL reconstruction through modulation of fibroblast to myofibroblast trans-differentiation by promoting PGE2 secretion.Bioact Mater. 2025 Jun 17;52:474-491. doi: 10.1016/j.bioactmat.2025.06.019. eCollection 2025 Oct. Bioact Mater. 2025. PMID: 40599342 Free PMC article.
References
-
- Thorpe CT, Screen HR, Tendon Structure and Composition. Advances in experimental medicine and biology 920, 3–10 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

