The TLR3/IRF1/Type III IFN Axis Facilitates Antiviral Responses against Enterovirus Infections in the Intestine
- PMID: 33203755
- PMCID: PMC7683398
- DOI: 10.1128/mBio.02540-20
The TLR3/IRF1/Type III IFN Axis Facilitates Antiviral Responses against Enterovirus Infections in the Intestine
Abstract
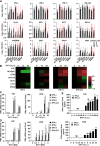
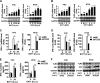
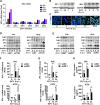
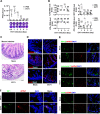
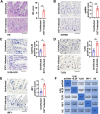
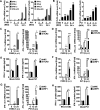
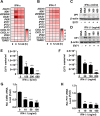
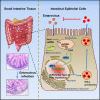
Enteroviruses infect gastrointestinal epithelium cells, cause multiple human diseases, and present public health risks worldwide. However, the mechanisms underlying host immune responses in intestinal mucosa against the early enterovirus infections remain elusive. Here, we showed that human enteroviruses including enterovirus 71 (EV71), coxsackievirus B3 (CVB3), and poliovirus 1 (PV1) predominantly induce type III interferons (IFN-λ1 and IFN-λ2/3), rather than type I interferons (IFN-α and IFN-β), in cultured human normal and cancerous intestine epithelial cells (IECs), mouse intestine tissues, and human clinical intestine specimens. Mechanistic studies demonstrated that IFN-λ production is induced upon enterovirus infection through the Toll-like receptor 3/interferon regulatory factor 1 (TLR3/IRF1) signaling pathway in IECs. In turn, the supplementation of IFN-λ subsequently induces intrinsically antiviral responses against enterovirus replication. Notably, intraperitoneal injection in neonatal C57BL/6J mice with mouse recombinant IFN-λ2 protein represses EV71 replication and protects mice from viral lethal effects. Altogether, these results revealed a distinct mechanism by which the host elicited immune responses against enterovirus infections in intestine through activating the TLR3/IRF1/type III IFN axis. The new findings would provide an antiviral strategy for the prevention and treatment of enterovirus infections and associated diseases.IMPORTANCE Enterovirus infections are significant sources of human diseases and public health risks worldwide, but little is known about the mechanism of innate immune response in host intestine epithelial surface during the viral replication. We reported the epithelial immune response in cultured human normal and cancerous cells (IECs), mouse tissues, and human clinical intestine specimens following infection with enterovirus 71. The results mechanistically revealed type III interferons (IFN-λ1 and IFN-λ2/3), rather than type I interferons (IFN-α and IFN-β), as the dominant production through TLR3/IRF1 signaling upon multiple human enterovirus infection, including enterovirus 71 (EV71), coxsackievirus B3 (CVB3), and poliovirus 1 (PV1). IFN-λ subsequently induced antiviral activity against enterovirus replication in vitro and in vivo. These studies uncovered the role of the novel process of type III IFN production involved in the TLR3/IRF1 pathway in host intestine upon enterovirus infection, which highlighted a regulatory manner of antiviral defense in intestine during enterovirus infection.
Keywords: Toll-like receptor 3; coxsackievirus B3; enterovirus; enterovirus 71; enterovirus infection; interferon regulatory factor 1; interferon-stimulated genes; intestine; intestine epithelial cells; neonatal C57BL/6J mice; poliovirus 1; type III interferons.
Copyright © 2020 Su et al.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
